Introduction
The understanding of ventilator graphics is a major void in our training. The title of this article suggests that it is about interpretation of the waveforms displayed on modern ICU ventilators. But suppose it was about interpretation of ECG waveforms. In that case the reader would probably recognize the importance of the topic and agree that to understand the waveforms, one would first have to understand the underlying cardiac physiology including the relevant terminology. Next, one would have to learn how waveforms reflect the underlying physiology and be able to classify them as normal or abnormal. And to make use of the interpretation for determining treatment, one would have to know how to assess the goal of treatment for a given patient. That knowledge would then be paired with a knowledge of drug taxonomy so that the mechanisms of action and indications for many different drugs can be discerned. Finally, one would have to know how to select the most appropriate drugs to meet the therapeutic goal.
It stands to reason then, that learning about ventilator waveform interpretation will involve first understanding the technical aspects of patient-ventilator interaction including ventilator design and the associated terminology. Next, one would have to learn how waveforms reflect the underlying patient-ventilator interaction and be able to classify them as normal or abnormal. And to make use of the interpretation for determining proper ventilator management, one would have to know how to assess the goal of ventilation for a given patient. That knowledge would then be paired with a knowledge of ventilator mode taxonomy so that the mechanisms of action and indications for many different modes can be discerned. Finally, one would have to know how to select the most appropriate mode to meet the therapeutic goal. Mastering these skills is imperative to appropriately manage mechanical ventilation.
This is a tall order, and unfortunately there was no generally accepted standard approach to interpreting ventilator waveforms as there is for ECG waveforms. However, our training program at the Cleveland Clinic has developed such a standardized procedure.1 This article will describe that procedure, the knowledge required to use the procedure, and provide references for further study.
Step 1: Identify the Mode of Ventilation
At last count2 there were nearly 500 different names of modes on the ventilators use in the United States. Yet these names (coined by ventilator manufacturers) represent maybe 50 different modes of ventilation using the mode taxonomy (classification scheme).2,3 Hence, the problem with identifying modes is similar to the problem of distinguishing brand names of drugs from generic names. As it turns out, this issue is important not only for waveform interpretation but for ordering and charting modes in modern electronic health records.
We define a mode as a predetermined pattern of patient-ventilator interaction. As this is an overview article, we do not have the space for a complete description of the mode taxonomy. Suffice it to say that any mode can be described in terms of three characteristics: the control variable, the breath sequence, and the targeting schemes.
Control Variable
A fundamental concept that serves as a foundation of understanding ventilator graphics is a simple mathematical model called the Equation of Motion for the respiratory system. Not only does it provide a conceptual framework for mode classification, but it can (and should) be used to explain every physical aspect of patient-ventilator interaction. This includes identification of effects of disease states, synchrony problems using waveform analysis and determination of optimal mode settings). Its broad utility makes the Equation of Motion a powerful model and well worth the effort to master its use. The equation has many forms, but for the purposes of creating a mode taxonomy we will use a simple one:
𝑃𝑣𝑒𝑛𝑡+𝑃𝑚𝑢𝑠=𝐸×𝑉+𝑅×𝑉̇
In this equation, P (pressure), V (volume), and V̇ (flow) are functions of time (t, implied) whereas E and R are constants (assumed to be unchanging during an inflation). Ventilator waveforms are simply graphs of the P, V, and V̇ functions. In this equation, Pvent is the pressure generated by the ventilator to create inspiratory flow and deliver the tidal. Pmus is the pressure generated by the patient’s muscles to generate inspiratory flow and tidal volume. The equation shows that for a patient receiving ventilatory assistance, Pvent and Pmus can work together, essentially sharing the total work of inspiration. E is the elastance of the respiratory system (E=∆P/∆V), the reciprocal of compliance (C = ∆V/∆P). V is volume, and the volume delivered during a single inspiration, the (maxim value of V as a function of time) is the tidal volume, VT;. R is the resistance of the respiratory system (R =∆P/V̇) and V̇ is inspiratory flow.
What does the equation of motion have to do with classifying modes? It says that if the ventilator controls one side of the equation for a particular mode, then the other will vary according to the respiratory system elastance and resistance. If we control the right side, this is called volume control (VC). By “control” we mean that both tidal volume and inspiratory flow are preset by you as the operator. Once V and V̇ are preset, Pvent is dependent on E and R. Some modes have preset tidal volume but not flow, and hence are not volume control but a form of pressure control with adaptive targeting (explained below). Some modes have preset inspiratory flow but not tidal volume, and again this is not volume control, but a form of pressure control. You might be wondering why we call it volume control when we could have called it flow control. We think it is for historical reasons: early volume control ventilators used pistons whose volume was adjusted to set the tidal volume. Nowadays, most ventilators have flow control valves to set tidal volume, but the use of “volume control” has persisted.
In contrast to VC, pressure control (PC) means that the left-hand side of the Equation of Motion is preset. In the simplest case, the pressure change above PEEP, which we will call the inspiratory target pressure, is preset. A more complicated case of pressure control occurs when the ventilator controls inspiratory pressure such that it is proportional to the patient’s inspiratory effort. One example of this is the mode called Proportional Assist Ventilation (PAV), where the ventilator detects inspiratory effort as the volume and flow generated by the patient. Another common example is the mode called Neurally Adjusted Ventilatory Assist (NAVA), where the ventilator detects inspiratory effort as an electrical signal generated by diaphragmatic contraction.
In summary, for VC modes the ventilator controls the shape of the volume waveform and for PC modes the ventilator controls the shape of the PC waveform.
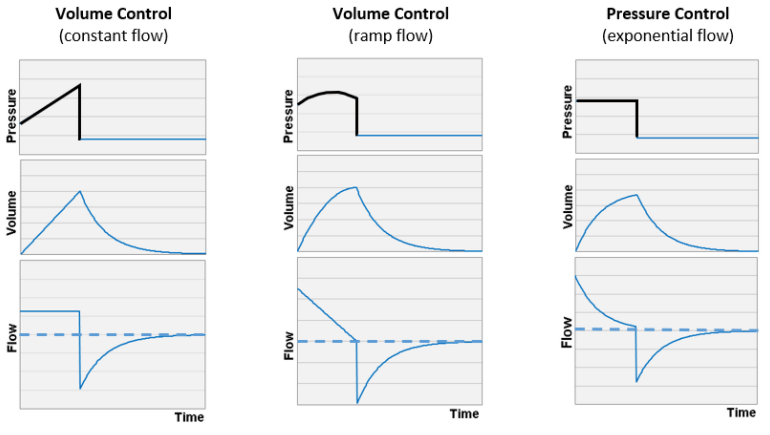
Given that summary, how do we distinguish VC from PC based on waveforms? The easiest path is to observe the flow waveform, if it has a geometric shape (rectangle or triangle) and does not change with effort (or changes in E or R) then the flow is controlled, i.e. it is VC. Figure 1 shows idealized waveforms for both. Identification of these flow waveforms constitutes a “basic” skill needed to appropriately manage mechanical ventilation.
Figure 1. Idealized graphic representations of ventilator pressure, volume, and flow waveforms.
Breath Sequence
A mode of ventilation can be thought of as a sequence of breaths delivered from a black box (the ventilator), like a chain of pearls on a necklace. In this construct, there are two kinds of breath (like white and black pearls). We define these two kinds of breaths in terms of how much control the patient has over the timing of inspiratory flow. This is important because timing is essential in determining patient-ventilator synchrony. Intuitively, we can say that a spontaneous breath as one for which the patient has total control over the start and stop of inspiratory flow, thus having complete control over breath frequency and inspiratory time. Starting inspiration is called the trigger event and stopping inspiration is called the cycle event. A spontaneous breath is formally defined formally as one for which inspiration is triggered and cycled by the patient. It follows that a mandatory breath is one for which the ventilator either triggers or cycles inspiration (or both). There are three possibilities for a mandatory breath: patient triggered and machine cycled, machine triggered and patient cycled, or machine triggered and machine cycled. There are modes that use each one of these possibilities. Some authors give different names to these possibilities, but that is an unnecessary complication for the purposes of a mode taxonomy.
Given that there are only two kinds of breaths, there can be only three breath sequences: all mandatory breaths (called continuous mandatory ventilation, CMV), mandatory and spontaneous breaths are possible (called intermittent mandatory ventilation, IMV), and all spontaneous breaths (called continuous spontaneous ventilation, CSV).
Types of IMV
At this point we must make an important distinction regarding the different types of Intermittent Mandatory Ventilation. This is because they account for a lot of the variety in modes and because they represent a major advance in the technical evolution of modes.
IMV(1): Historically, Intermittent Mandatory Ventilation (IMV) started out as a patient circuit modification made by clinicians because ventilators (ie, adult ventilators) only provided Continuous Mandatory Ventilation (CMV). Note that infant ventilators at this time only provided PC-IMV and this was because in those days (early 1970s) the technology was not available to accurately trigger mandatory breaths for infants that often have erratic inspiratory efforts. Adult ventilators were modified by inserting anesthesia bags and one-way valves in the patient circuit and supplying the bags with a continuous flow of air/oxygen to support spontaneous breathing between the mandatory breaths. The idea was that to wean patients, all you had to do was gradually decrease the mandatory breath rate and the patient would maintain minute ventilation by increasing the spontaneous breath rate. It was not long before ventilator manufacturers started to build this feature into their modes. We call this IMV(1) where mandatory breaths are delivered at the set rate regardless of what the patient does. An example would be a mode called SIMV Pressure Control (PB 980 ventilator), However, there are data to suggest that, for most patients, IMV(1) prolongs the weaning process compared to daily spontaneous breathing trials followed by sudden discontinuation of ventilation.
IMV(2): Makers of home care ventilators recognized that their patients’ comfort was an important goal. Mandatory breaths are not as comfortable as spontaneous breaths because an arbitrary pre-set frequency and inspiratory time is imposed on the patient. Yet mandatory breaths provided the safety -net in the event of apnea. Hence, engineers invented a compromise; if the patient’s spontaneous breath rate exceeds the set mandatory breath rate, mandatory breaths would be suppressed. This is called IMV(2). An example would be a mode called Spontaneous/Timed (V60 ventilator manufactured by Philips). If mandatory breaths are indeed suppressed, the ventilator waveforms look like continuous spontaneous ventilation (CSV).
IMV(3): There is a drawback of IMV(2); If the patient’s lung disease worsens, often the breath pattern becomes rapid and shallow. Hence the patient may hypoventilate (due to a small VT and hence large VD/VT) while the ventilator continues to suppress the larger mandatory breaths. IMV(3) attempts to avoid this problem by suppressing mandatory breaths only if the spontaneous minute ventilation is more than the minute ventilation created by the preset mandatory breath rate and tidal volume. An example would be a mode called Mandatory Minute Volume (V500 ventilator manufactured by Draeger). Of course, this is only a partial solution because the settings for frequency and tidal volume are just a guess by the clinician about gross minute ventilation, not knowing the true alveolar minute ventilation requirement. More advanced modes, like IntelliVent, use capnography to automatically set an appropriate minute ventilation to maintain acceptable ETCO2.
IMV(4): Finally, the risk of asynchrony during conventional volume control is high because the operator sets arbitrary values for tidal volume and inspiratory flow. For a patient making a large enough inspiratory effort, patient-ventilator asynchrony may occur in the form of potentially dangerous work shifting. The engineering solution is for the ventilator to recognize this condition (as a drop in airway pressure below a preset threshold) and compensate. Recall from the equation of motion that in volume control, if Pmus (inspiratory effort) increases, then Pvent (airway pressure) must decrease an equal amount. Thus, the ventilator monitors Pvent, and if it drops by some default threshold (eg, 3 cm H2O) the ventilator gives as much flow (and hence volume) as the patient wants. If the effort is large enough, inspiration may also change from volume/time cycling to flow cycling. This is a form of dual targeting, where volume control switches to pressure control and the tidal volume is larger than the preset value. In this case, if inspiration becomes both patient triggered and patient cycled, it is by definition a spontaneous breath that is obviously more synchronous with the patient’s demand. This is called IMV(4), where individual mandatory breaths may be suppressed by spontaneous breaths if the inspiratory effort is high enough. An example would be a mode called Volume Control with Flow Adaptation (Servo-U ventilator manufactured by Getinge).
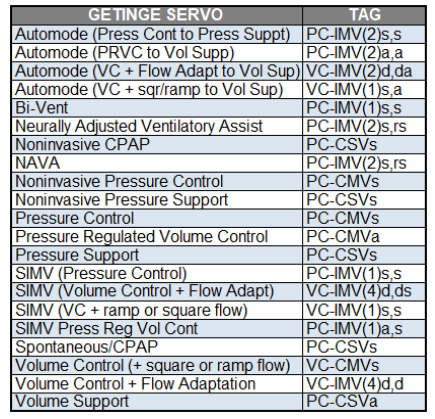
Recognizing the type of IMV from athe ventilator’s graphical displays is often difficult. It may be possible if you spend enough time observing the patient. A more practical approach is to simply read the description of the mode in the ventilator’s operator manual or looking up the mode in a classification table (Table 1)
Table 1. Example look-up table that gives mode names and mode classifications for a common ventilator (Servo-U Getinge).
Breath Sequence
Targeting Scheme
A targeting scheme is the software that connects the operator inputs and ventilator outputs to achieve a specific ventilatory pattern. Think of it as the program running the mode. The targeting scheme is a key component of a mode description. Thus, a target is a predetermined goal of ventilator output.
Currently, there are at least seven different targeting schemes used on commercially available ventilators.3 They are described below, with the lower case letters that represent them in abbreviated form in parentheses:
- Set-point (s): The operator sets all parameters of the pressure waveform (pressure control modes) or volume and flow waveforms (volume control modes). The advantage is simplicity. The disadvantage is that changing patient condition may make the settings inappropriate, making frequent adjustment necessary. An example mode name is Assist/Control.
- Dual (d): The ventilator can automatically switch between volume control and pressure control during a single inspiration. The advantage is the ability to adjust to changing patient condition and assure either a preset tidal volume or peak inspiratory pressure, whichever is deemed most important. The disadvantage is that some forms are complicated, difficult to set, and need constant readjustment. This is usually not present as a mode per se, but as a function that changes the behavior of the mode. The example is Flow adaptation on the Servo-U ventilator.
- Bio-variable (b): The operator sets a target inspiratory pressure and a percent variability from 0% to 100%. The ventilator then varies the inspiratory pressure target breath by breath randomly. The advantage is varying tidal volume breath by breath to mimic normal breathing may improve gas exchange. The disadvantage is that it is currently available in only one mode, Variable Pressure Support, on the Dräger V500 ventilator.
- Servo (r): The operator sets the level of support and the ventilator automatically adjusts the inspiratory pressure to be in proportion to the patient’s inspiratory effort. The advantage is that the more assistance the patient demands, the more support the ventilator delivers. No other targeting scheme does this. The disadvantage is that it requires estimates of artificial airway and respiratory system mechanical properties or special equipment to monitor the respiratory effort signal. Example mode names include Automatic Tube Compensation, Proportional Assist Ventilation, and Neurally Adjusted Ventilatory Assist.
- Adaptive (a): The operator sets a target tidal volume and the ventilator automatically sets inspiratory pressure target. The advantage is that minute ventilation is stabilized with varying respiratory system mechanics (resistance, compliance, and inspiratory effort). The disadvantage is that the ventilator may decrease support to a dyspneic patient because it cannot tell the difference between improving compliance and increasing inspiratory effort. The first mode to use this was called Pressure Regulated Volume Control.
- Optimal (o): The ventilator automatically adjusts the targets of the ventilatory pattern to minimize or maximize some desired variable. The only commercially available example to date is one designed to minimize the transfer of power from the ventilator to the patient.4 The advantage is that it can adjust to a changing patient condition. The disadvantage is that the automatic adjustment may be inappropriate if the algorithm assumptions are violated or they do not match the patient’s actual physiology. The only modes currently using this are Adaptive Support Ventilation (Hamilton ventilators) and Adaptive Ventilation Mode (Vyaire bellavista ventilator).
- Intelligent (i): This targeting scheme uses artificial intelligence programs such as rule-based expert systems. The advantage is that it can adjust to a changing patient condition in a fashion that mimics human decision making. The disadvantage is that the automatic adjustment may be inappropriate if the algorithm assumptions are violated or they do not match the patient’s actual physiology. The only modes currently using this are SmartCare/PS and IntelliVent-ASV.
Step 2: Determine the Load
In the Equation of Motion, the inspiratory force (Pvent) is opposed by two other forces we call loads. The term E×V has the dimensions of pressure and is called the elastic load. As E or V increase (or C decreases) the load increases and it takes more inspiratory force (pressure) to inflate the respiratory system to the same VT. In practice, an abnormal elastic load is generally due to worsening disease that decreases compliance.
The second opposing force, consists of the term R×V̇ which has the dimensions of pressure also and is called the resistive load. As R or V̇ increase, the load increases and more inspiratory pressure is needed for inflation. In practice, an abnormal resistive load is generally due to worsening disease that increases airway resistance (or perhaps the need to suction the artificial airway).
In order to determine the load, there must be little or no interference by Pmus, otherwise we cannot assess E and R accurately.
Figure 2. Left-inspiratory effort (Pmus > 0) distorts volume and flow waveforms for a pressure control breath. Right-inspiratory effort distorts pressure waveform for a volume control breath.
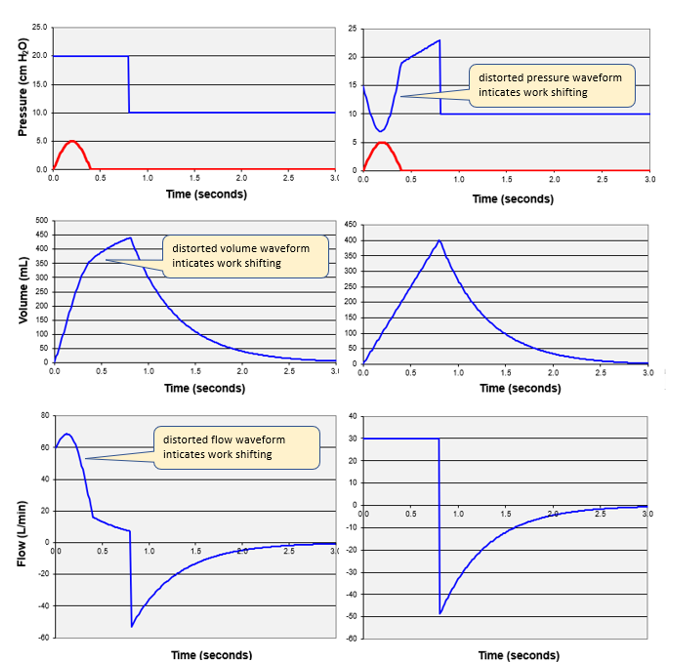
But all is not lost. We can get some important clues by looking at expiration. Expiration is generally passive and as such we can look at the shape of the exponentially decreasing flow or volume waveforms (see Figure 3).
Figure 3. The time constant curves.
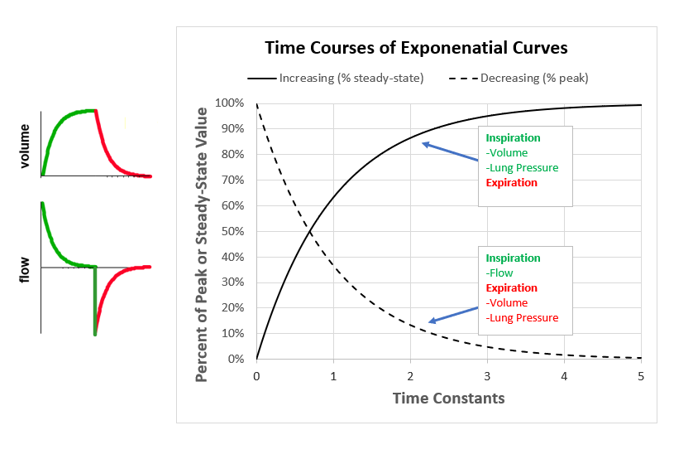
The rate at which an exponential curve increases orf decreases is determined by R and C. The product or R multiplied by C is called the time constant, which has units of time (seconds). The time constant (symbolized by the Greek letter τ) is defined as the period during which the function changes by 63%. Starting from peak expiratory flow, in the period of one τ, flow decays by 63% (ie, to 37% of the peak value). In the next τ, flow decays another 63% (to 14%). Exhalation is considered complete after 5τ when flow has decayed to 1% (theoretically the curve never reaches 0%), but often we assume 3τ (ie, 5%) is close enough to avoid significant autoPEEP. Note that the expiratory flow and volume waveforms have the same shape. The time constant has the added utility that it describes passive inflation as well. To use τ for waveform interpretation, we must first know what a normal value is. Assuming values5 for a normal intubated adult: R = 10 cm H2O/L/s and C = 0.050 L/cm H2O, it is easy to remember that τ = 0.5 seconds and normal expiration takes about 1.5 seconds (3τ). Remembering what we said above about abnormal values of τ, if expiration is shorter than 1.5 seconds we suspect decreased C and if it is longer than 1.5 seconds, we suspect increased R. Flow waveforms with normal, short, and long time constants are illustrated in Figure 4.
Figure 4. Look at the flow waveforms. Left is normal τ (R = 10, C = 50); middle is long τ (R = 20, C = 50); right is short τ (R = 10, C = 35).
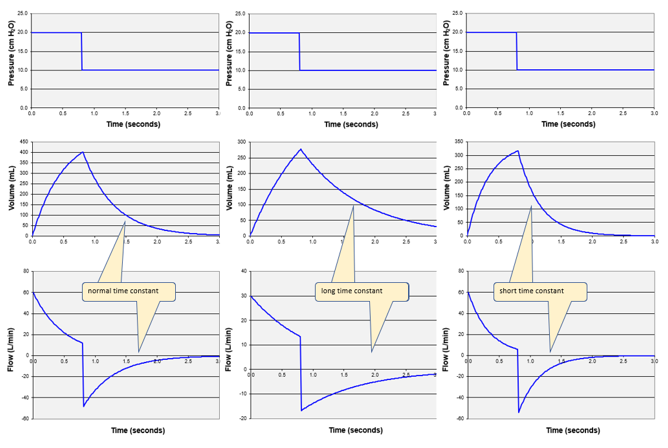
In summary, under passive conditions, for VC modes, we determine the load by looking at the inspiratory pressure waveform. For PC modes, we look at the inspiratory flow waveform. If inspiratory waveforms are distorted by Pmus, we look at the expiratory flow and determine if the time constant is abnormal. A long time constant is generally due to increased R and a short time constant is usually due to a decreased C.
Step 3: Diagnose the Patient-Ventilator Interaction Status
In order to interpret ventilator waveforms in terms of patient-ventilator interaction status, we must first introduce a taxonomy (classification system) for diagnosing synchrony states.1 The hierarchical structure of this taxonomy is based on the phases of a breath: trigger (start of positive flow), inspiration (period from start of positive flow to start of negative flow), cycle (start of negative flow), expiration (period from start of negative flow to start of positive flow). Recall that positive flow indicates inspiration and negative flow indicate expiration.
Trigger
Normal: Inspiration starts within an acceptable delay (eg, 100 ms) after start of patient inspiratory effort (Figure 5)
Figure 5. Normal trigger event (volume control inspiration)
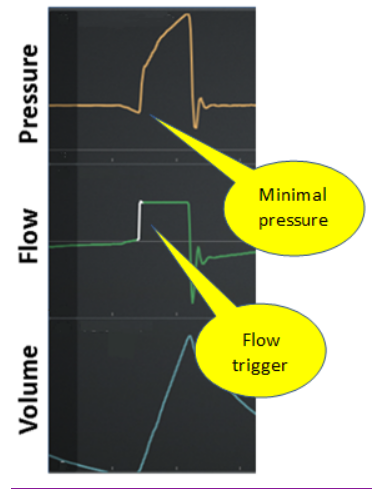
Early: Inspiratory effort appears after the machine triggers inspiration. Also called “reverse trigger” Figure 6)
Figure 6. Early trigger (machine trigger then patient effort)
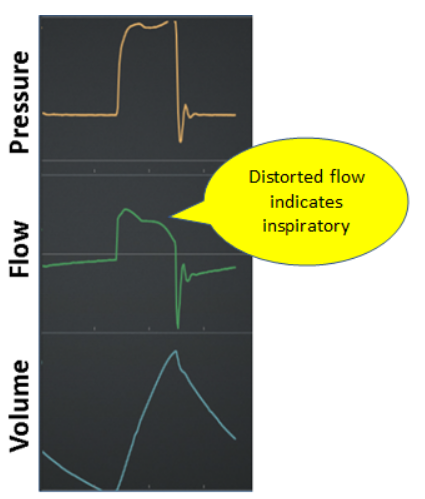
Late: Inspiration starts beyond an acceptable delay (eg, 100 ms) after start if inspiratory effort (Figure 7)
Figure 7. Late trigger
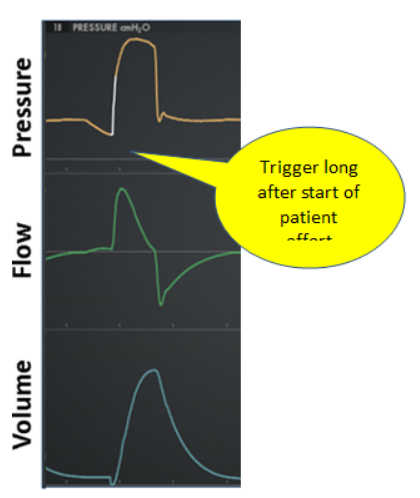
False: A trigger event not caused by patient effort (Figure 8)
Figure 8. False trigger
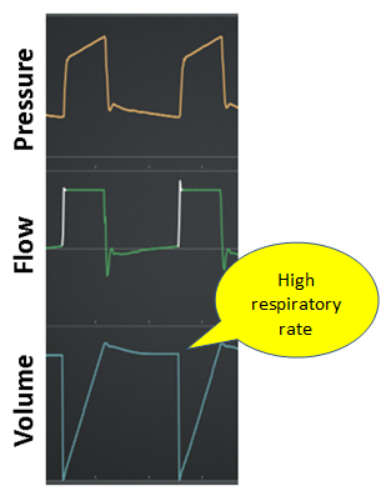
Failed: A failed trigger effort means that the inspiratory signal (eg, pressure or flow change) generated by the patient was not recognized by the ventilator (Figure 9)
Figure 9. Failed trigger effort
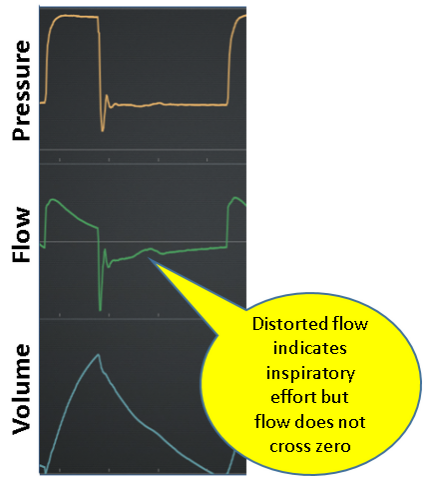
Inspiration
Normal: Inspiration is passive (ie, no inspiratory effort).
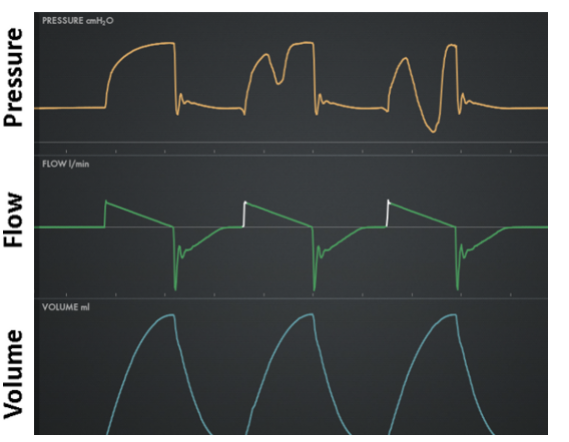
Work shifting: Inspiration is active (ie, presence of inspiratory effort) resulting in the patient performing some portion of the total work of inspiration. Work is the result of volume being delivered under pressure (eg, higher pressure or volume means higher work per breath). From the Equation of motion, we see that for VC, if Pmus increases then Pvent must decrease an equal amount, total work stays the same (ie, total pressure and VT are the same), but some work has shifted to the patient due to increased Pmus and same VT. For PC, when Pmus increases, Pvent stays the same but VT increases, the total pressure increases (ie, Pmus adds to Pvent) but work per liter of volume remains the same so that again some work has shifted to the patient due to higher Pmus and higher VT. Figure 10 shows work shifting in volume control and Figure 11 shows work shifting in pressure control.
Figure 10. Work shifting in volume control: left is passive (no work shifting), middle is moderate, right is severe (flow starvation)
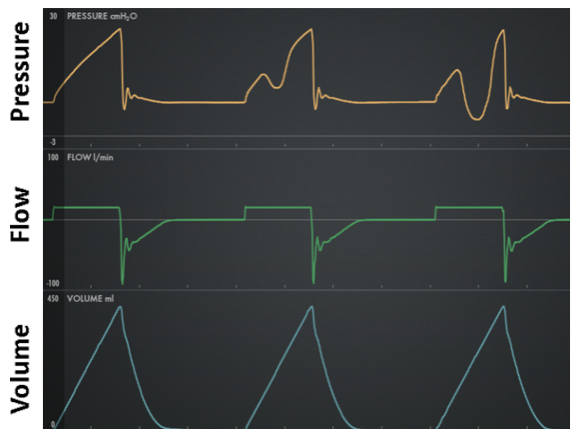
Figure 11. Work shifting in pressure control: left is passive (no work shifting), middle is moderate, right is severe (flow starvation)
Cycle
Normal: Inspiratory flow ends within an acceptable period around the point where Pmus peaks. Peak Pmus must be inferred from the pressure waveform (VC) or the flow waveform (PC).
Early: Inspiratory flow ends before Pmus peaks (Figure 12). Peak Pmus must be inferred from the pressure waveform (VC) or the flow waveform (PC).
Figure 12. Early cycle
Late: Inspiratory flow ends after Pmus peaks (Figure 13). Peak Pmus must be inferred from the pressure waveform (VC) or the flow waveform (PC).
Figure 13. Late cycle
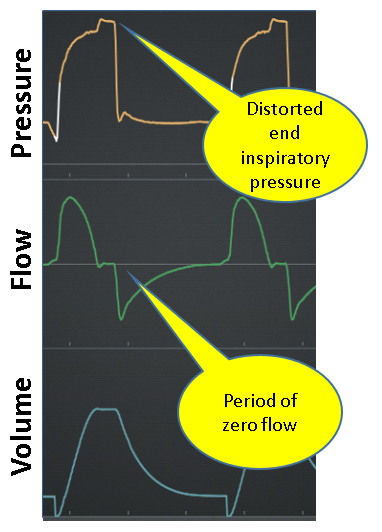
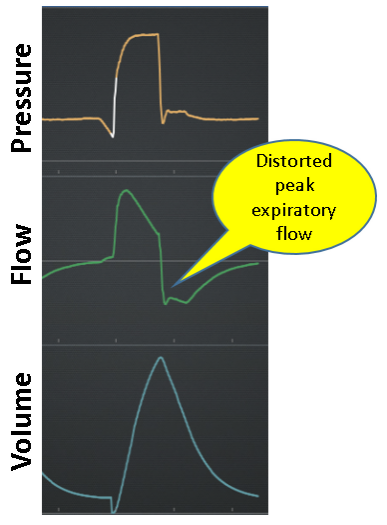
Expiration
Normal: Expiration is passive (ie, no inspiratory effort) and shows smooth exponential decay of flow from peak expiratory flow to zero (see Figure 13).
Expiratory work: Expiration is active (ie, evidence of expiratory effort) as indicated by increased flow compared to passive expiration (Figure 14).
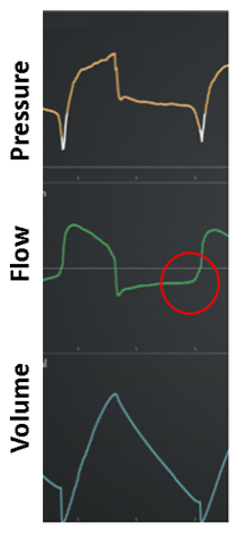
Step 4: Intervention
Any medical treatment can be summarized as a process of assessing the needs of the patient to determine the goal of treatment, taking inventory of the available treatment options, and finally matching the best treatment to the need. In the context of mechanical ventilation, there are only three basic goals: safety (adequate gas exchange and lung protection from tidal volume overdose), comfort (optimum patient-ventilator synchrony) and liberation (minimize duration of ventilation). Having identified the mode of ventilation and synchrony problems using the steps above, the clinician must decide which of the three goals takes precedence at the time of the assessment. Having done that, and being familiar with all the modes on the available ventilators, the clinician is able to select one mode as most likely to serve the goal of ventilation at that time. This procedure has been described in detail previously.7 Once the mode is selected, the specific settings for that mode must be optimized.
Of note, for a patient with very high ventilatory drive (as is common with patients ventilated for COVID-19) resulting in extreme work shifting, no mode or mode setting can fix the problem. The reason for the high drive must be determined and treated, which may include sedation and paralysis.
Conclusion
We hope the reader appreciates that interpretation of ventilator graphics is a complex skill, relying on a large amount of assumed knowledge about respiratory system mechanics, ventilator design, the taxonomy of modes of ventilation and the taxonomy of patient-ventilator interaction. Because synchrony problems are associated with worse outcomes and fewer hospital-free days,8 skill in recognizing and treating these problems should improve patient care.
Resources
We have developed an educational program for clinicians called SEVA (pronounced “say-va” which stands for Standardized Education for Ventilatory Assistance. To support this program we conduct a free online seminar every two weeks (called SEVA-VentRounds). This is a live, interactive analysis (conducted by the authors) of actual ventilator screen shots using the approach described above. VentRounds is open to anyone. More information can be found, here.
Another resource we have created to support SEVA is a software simulator of patient-ventilator interaction. This is what we used to create Figures 2 and 4 above. This simulator has a generic ventilator interface and allows setting respiratory system mechanics (ie, resistance, compliance, and inspiratory effort) and show both waveforms (pressure, volume, and flow) along with a host of calculated parameters (including work and power). This software is free and runs on any computer that has Microsoft Excel. Find it, here.
References
- Mireles-Cabodevila E, Siuba MT, Chatburn RL. A Taxonomy for Patient-Ventilator Interactions and a Method to Read Ventilator Waveforms. Respir Care. September 2021:respcare.09316.
- Volsko TA, Chatburn RL, El-Khatib MF. Equipment for Respiratory Care. 2nd ed. Burlington MA: Jones & Bartlett Learning; 2022.
- Chatburn RL, El-Khatib M, Mireles-Cabodevila E. A taxonomy for mechanical ventilation: 10 fundamental maxims. Respir Care. 2014;59(11):1747-1763.
- van der Staay M, Chatburn RL. Advanced modes of mechanical ventilation and optimal targeting schemes. Intensive Care Med Exp. 2018;6(1):30.
- Arnal JM, Garnero A, Saoli M, Chatburn RL. Parameters for simulation of adult subjects during mechanical ventilation. Respir Care. 2017;63(2):158-168.
- Chatburn RL, Mireles-Cabodevila E. 2019 Year in Review: Patient-Ventilator Synchrony. Respir Care. 2020;65(4):558-572.
- Mireles-Cabodevila E, Hatipoglu U, Chatburn RL. A rational framework for selecting modes of ventilation. Respir Care. 2013;58(2):348-366.
- Zhou Y, Holets SR, Li M, et al. Etiology, incidence, and outcomes of patient-ventilator asynchrony in critically-ill patients undergoing invasive mechanical ventilation. Sci Rep. 2021;11(1):12390.
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.