You can find inhaled pulmonary vasodilators (iPVD) provided across our health care continuum. For example, you can see IPVDs in use from the home management of pulmonary arterial hypertension (PAH) through the intra-operative environment. The delivery of iPVDs is a rapidly expanding service in respiratory therapy and requires close attention for best practice.
The ability to affect pulmonary vascular resistance (PVR) without impacting the systemic vasculature has produced improvements in both morbidity and mortality in our patients.1,2 Other noted effects of these agents have expanded indications for use regarding reperfusion injury, sickle cell disease, refractory hypoxemia, and right heart dysfunction.3,4,5 From infants to adults, targeted pulmonary vascular therapy has demonstrated improvements for both oxygenation and cardiac function.
Nitric Oxide by inhalation (iNO) was the first FDA-approved iPVD. Arriving on the clinical scene in 1999, this agent received approval for neonatal patients (>34 weeks gestation) in hypoxic respiratory failure with pulmonary hypertension.5 The benefits of lowering PVR demonstrated improvements in oxygenation and right heart function. This physiologic impact quickly found iNO incorporated into the management of other congenital cardiac anomalies, adult cardiac surgery, and at times as rescue therapy in adults for refractory hypoxemia.2,6 Although iNO use in adults does not carry FDA approval, subsequent research and practice paterns has resulted in significant improvements in the care for chronic and acute patients suffering pulmonary hypertension and right ventricular dysfunction.3
Today, targeted PVR therapy comes in several forms. To appreciate the current state of iPVDs, we will review the current knowledge of vascular tone modulation and the available pharmacological agents used. We will also focus on the inhaled pulmonary vasodilators.
The modulation of vascular tone occurs through three identified mechanisms: the nitric oxide pathway, endothelin pathway, and prostacyclin pathway. Nitric oxide is an endogenous compound formed in the vascular endothelial cells, and its release stimulates the development of cGMP, which produces vasodilation.7 Prostacyclin’s or their derivatives are also produced in the vascular endothelium and stimulate cAMP-producing vasodilation.7 Endothelin (ET-1) nets vasoconstriction, and it too is typically produced in the vascular epithelium.7
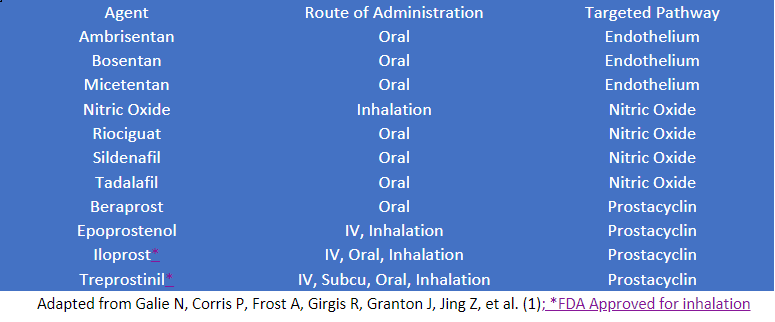
These three autonomic mechanisms control the arteriole tone and are the focus of our pharmacological interventions. There are approximately 10 available agents.1 These agents are administered by inhalation, oral, subcutaneously, and intravenously. Targeting one or more of the pathways is a common approach to therapy. We routinely use systemic administration as first-line treatment for patients with PAH. Still, we also use it as adjunctive therapies where pulmonary hypertension and right heart dyisfunction are a clinical challenge. Pulmonary vasodilator agents, their route of administration, and their targeted pathway include:
Systemic therapy may not achieve the desired goal, be optimal for patient compliance, and produce undesired side effects.8 Furthermore, this treatment may require additional therapies.8
Inhaled pulmonary vasodilators are an option. These agents have a rapid onset of action with minimal side effects, making them recommended therapies in the management of chronic PAH.5
There are only two FDA-approved inhaled pulmonary vasodilators (iPVD) used in this manner. They operate through the prostacyclin pathway: iloprost (VENTAVIS) and treprostinil (TYVASO).1 These agents are recommended by inhalation for the treatment of patients with advanced PAH (World Health Organization (WHO) functional class (FC) III-IV).10 The administration of these agents by inhalation is intermittent (4-8 daily sessions) and done through proprietary devices. (Fig 1, 2) Iloprost is delivered through the I-NEB ADAPTIVE AEROSOL DELIVERY SYSTEM (Philips Healthcare, Andover, MA, USA), and treprostinil is delivered through the TYVASO INHALATION SYSTEMS (TD-100 or Optineb; United Therapeutics, Research Triangle Park, NC, USA).10 These agents may cause bronchial irritation resulting in bronchospasm.27 Pre-treatment with a beta-agonist may be warranted in some individuals. Both of these agents have been investigated for off label use in both pediatrics and adults. The focus of these investigations included safety, tolerability, oxygenation, and the progression of ARDS due to the prostacyclin cytokine inhibitory function.5,9,10,11
Figure 1 & Figure 2

Acute management of pulmonary hypertension is clinically seen in critical care and perioperative environments. In the setting of high PVR, pulmonary vasodilation produces improvements in oxygenation and improves blood flow through the lungs. This process improves the pulmonary venous return to the left heart, optimizing cardiac output. Lowering PVR decreases the right ventricular afterload. In the setting of right heart dysfunction, it can improve right ventricular outflow and optimize cardiac output. These improvements suggest that using PVDs in these settings may improve refractory hypoxemia and acute right heart failure, benefits that can minimize ventilator support, and the need for mechanical cardiac devices. Inhaled PVDs are often seen in these settings.4,6,12
iPVDs as a first-line agent is through continuous administration and is reserved for the acute management of PH when systemic therapy is insufficient, impractical or the relative side effects of systemic therapy are not congruent with the care plan. Continuous iPVDs include inhaled nitric oxide and inhaled epoprostenol. Use of these agents is found routinely in the newborn’s respiratory distress, preoperatively for lung or heart transplantation, and ventricular assist device implantation. To a lesser extent, iPVDs are used for refractory hypoxemia. The two most commonly used agents for these purposes are inhaled nitric oxide (iNO) and inhaled epoprostenol (iEPO).5,13
Inhaled NO diffuses across the alveolar-capillary membrane and results in local dilation of the pulmonary vessels, thus decreasing PVR. With a rapid onset of action and rapid metabolism, iNO produces a local vasodilatory response. This result makes the agent a relatively ideal choice in managing PVR.14 Delivery of iNO is through large proprietary devices that require an additional degree of technical skill. Clinical dosing has been reported to range from 10 to 20ppm.15 The lower doses are seen during weaning. Rebound pulmonary hypertension has been identified when nitric oxide is abruptly removed. Incremental weaning and monitoring should be performed when proceeding with discontinuation.3 The long-term effects of iNO have suggested a degree of renal toxicity, but these reports are suggestive in nature only.16 The cost and labor associated with these administration devices has stimulated the search and use of alternative agents.17
Inhaled epoprostenol (iEPO) made a profound entry in the clinical stage when De Wet et al (2003) reported the safety and efficacy of iEPO in a clinical series of 126 cardiac surgery patients. In this clinical series, the authors reported benefits to pulmonary artery pressures without systemic effects and potential cost savings to their institution of nearly $680,000 during their two-year study period.18 Since then, iEPO has been studied in several trials as a viable, cost-effective alternative to iNO.19,20
A recently ended clinical trial (NCT0308152) was a triple blinded study of 424 patients receiving lung transplantation, heart transplantation, and LVAD insertion comparing iEPO to iNO.21 These results may have important implications regarding the choice of agents. IEPO is delivered through continuous nebulization, requiring a continuous infusion pump.
The delivery of this agent has three formulations that require consideration. These are; Veletri©, EPOPROSTENOL SODIUM, and FLOLAN©. VELETRI© is arginine-based and is a less viscous formulation, making it suitable for nebulization with a vibrating mesh nebulizer. FLOLAN© and EPOPROSTENOL SODIUM are glycine-based formulations, much more viscous and have been reported to cause ventilator malfunction.28 This difference may impact choice of nebulizer for delivery which could further impact mechanical ventilation function.22 These agents are not approved for inhalation by the Food and Drug Administration and are used off-label.
The search for optimal PVR management is an ongoing process and deserves other considerations beyond the medication alone. We assume that the optimal delivery of these agents is to the alveolar-capillary membrane, thus producing alveolocapillary vasodilation. However, it has been suggested by Grava studying the concentration effect of both inhaled and instilled milrinone that the deposition site may be more influential on response.23 Vasodilation from milrinone occurs in the smooth muscle cells. Delivery of this agent at the precapillary arteriole level was important to its mode of action, which was demonstrated by simple jet nebulization providing the largest improvement in mean pulmonary arterial pressures.24 The safety profile of iEPO has been raised by others noting the unreliability of vibrating mesh nebulizers, added flow to ventilator circuits from jet nebulizers, lack of alarm systems for iEPO, and optimal dosing ranges have yet to be established in aerosolization.25
Despite these concerns, other agents by inhalation continue to be studied: sodium nitroprusside, nitroglycerin, and the phosphodiesterase inhibitors sildenafil, and levosimendan.5,17,26
We use inhaled pulmonary vasodilators in a variety of circumstances. Front line expert support of this technical and complex service by respiratory therapy is key to optimal outcomes. The wide range of iPVDs applications demands a sophisticated approach that is conscientious of the time, knowledge, and skills for best practice. Intermittent iPVD use in PAH has demonstrated benefits but is highly dependent on adherence due to the delivery devices and treatment plans. The respiratory therapist’s role for these patients must be respectful of the need for continuing education and care team support. Disease management, inclusive of respiratory therapy, is a high-value function for these patients. Continuous delivery of iPVDs is done through unique systems that require a high degree of technical expertise. Evidential support for equivalence of iEPO to iNO is growing, and its use as a cost-saving alternative is a valid consideration. Nitric Oxide is now available from a few distributors, evaluating optimum practice for continuous iPVD delivery at the forefront of respiratory departments.
Considerations in continuous iPVD delivery include iPVD agent, delivery system operation, the interaction of delivery system and respiratory support devices, the environment used, patient transport, monitoring, and training. The current iPVDs available for use have unique aspects to each agent and cannot be universally applied. Therefore, considerations in providing this service should include the individual needs of the patient population, the environment of use, and identified outcomes desired.
References
- Galie N, Corris P, Frost A, Girgis R, Granton J, Jing Z, et al. Updated treatment algorithm of pulmonary hypertension. Journal of the American College of Cardiology 2013;62(Suppl):D60-D72. doi.org/10.1016/j.jacc.2013.10.031
- Nelin L, Hoffman G. The use of inhaled nitric oxide in a wide variety of clinical problems. Pediatric Clinics of North America 1998;45(3):531-548. doi: 10.1016/s0031-3955(05)70026-2.
- Ichinose F, Roberts J, Zapol W. Inhaled Nitric Oxide: A selective pulmonary vasodilator current uses and therapeutic potential. Circulation 2004;109:3106-3111. doi: 10.1161/01.cir.0000134595.80170.62
- Rao V, Ghadimi K, Keeyapaj W, Parsons C, Cheung A. Inhaled nitric oxide (iNO) and inhaled epoprostenol (iPGI2) use in cardiothoracic surgical patients: is there sufficient evidence for evidence-based recommendations? Journal of Cardiothoracic and Vascular Anesthesia 2018;32:1452-1457. doi: 10.1161/01.cir.0000134595.80170.62
- Liu K, Wang H, Yu S, Tu G, Luo Z. Inhaled pulmonary vasodilators: a narrative review. Annals of Translational Medicine 2021;9(7):597. doi.org/10.21037/atm-20-4895
- Argenziano M, Choudri A, Moazami N, Rose E, Smith C, Levin H, et al. Randomized, double-blind trial of inhaled nitric oxide in LVAD recipients with pulmonary hypertension. The Society of Thoracic Surgeons 1998;65:340-345. doi: 10.1016/s0003-4975(97)01307-6
- Humbert M, Sitbon O, Simmonneau G. Treatment of pulmonary hypertension. The New England Journal of Medicine 2004;351(14):1425-1436. doi: 10.1056/NEJMra040291
- Lang I, Gaine S. Recent advances in targeting the prostacyclin pathway in pulmonary arterial hypertension. European Respiratory Review 2015;24:630-641. doi: 10.1183/16000617.0067-2015
- Ford H, Anderson W, Wendlandt B, Bice T, Ceppe A, Lanier J, Carson S. Randomized, placebo-controlled trial of inhaled treprostinil for patients at risk for acute respiratory distress syndrome. Annals of the American Thoracic Society 2021;18(4):641-647. doi: 10.1513/AnnalsATS.202004-374OC
- Winterhalter M, Simon A, Fischer S, Rahe-Mayer N, Chamtzidou N, Hecker H, et al. Comparison of inhaled iloprost and nitric oxide in patients with pulmonary hypertension during weaning from cardiopulmonary bypass in cardiac surgery: a prospective randomized trial. Journal of Cardiothoracic and Vascular Anesthesia 2008;22(3):406-413. doi:10.1053/j.jvca.2007.10.015
- Sawheny E, Ellis A, Kinasewitz G. Iloprost improves gas exchange in patients with pulmonary hypertension and ARDS. Chest 2013;144(1):55-62. doi: 10.1378/chest.12-2296
- Hoeper M, Granton J. Intensive care unit management of patients with severe pulmonary hypertension and right heart failure. American Journal of Respiratory and Critical Care Medicine 2011;184:1114-1124. doi: 10.1164/rccm.201104-0662ci
- Haj R, Cinco J, Mazer C. Treatment of pulmonary hypertension with selective pulmonary vasodilators. Current Opinion in Anesthesiology 2006;19(1):88-95. doi: 10.1097/01.aco.0000192765.27453.5a
- Griffiths M, Evans T. Inhaled nitric oxide therapy in adults. The New England Journal of Medicine 2005;353:2683-2695. doi: 10.1056/nejmra051884
- Hill N, Preston I, Roberts K. Inhaled therapies for pulmonary hypertension. Respiratory Care 2015:60(6):794-805. doi: 10.4187/respcare.03927
- Ruan S-Y, Wu H-Y, Lin H-H, Wu H-D, Yu C, Lai M-S. Inhaled nitric oxide and the risk of renal dysfunction in patients with acute respiratory distress syndrome: a propensity-matched cohort study. Critical Care 2016;20:389. doi: 10.1186/s13054-016-1566-0
- Lowson S. Inhaled alternatives to nitric oxide. Anesthesiology 2002;96(6):1504-1513. doi: 10.1097/00000542-200206000-00034
- De Wet C, Affleck D, Jacobsohn E, Avidan M, Tymkew H, Hill L, et al. Inhaled prostacyclin is safe, effective, and affordable in patients with pulmonary hypertension, right heart dysfunction, and refractory hypoxemia after cardiothoracic surgery. The Journal of Thoracic and Cardiovascular Surgery 2004;127(4):1058-1067. doi: 10.1016/j.jtcvs.2003.11.035
- Krebs R, Morita Y. Inhaled pulmonary vasodilators and thoracic organ transplantation: does evidence support its use and cost benefit? Seminars in Cardiothoracic and Vascular Anesthesia 2020;24(1):67-73. doi: 10.1177/1089253219870636
- Augoustides J, Ochroch E. Inhaled selective pulmonary vasodilators. International Anesthesiology Clinics 2005;43(2):101-114 doi: 10.1097/01.aia.0000157495.63367.2a
- ClinicalTrials.gov. Inhaled Selective Pulmonary Vasodilators for Advanced Heart Failure Therapies and Lung Transplantation Outcomes (INSPIRE-FLO) Clinical trial number NCT0308152. https://clinicaltrials.gov/ct2/show/NCT03081052?term=inspire+flo&draw=2&rank=1
- Dhand R. How should aerosols be delivered during invasive mechanical ventilation?Respiratory Care 2017;(62):10:1343-1367 doi: https://doi.org/10.4187/respcare.05803
- Gavra P, Denault A, Theoret Y, Perrault L, Varin F. Pharmacokinetics and pharmacodynamics of nebulized and intratracheal milrinone in a swine model of hypercapnia pulmonary hypertension. Journal of Cardiothoracic and Vascular Anesthesia 2018;32:2130-2138. doi.org/10.1053/j.jvca.2018.01.031
- Ghadimi K, Cappiello J. Intrapulmonary milrinone for cardiac surgery provides insight into precision delivery of aerosolized vasodilators. (editorial). Journal of Cardiothoracic and Vascular Anesthesia 2018;32:2139-2141. doi.org/10.1053/j.jvca.2018.03.009
- Cosa N, Costa Jr E. Inhaled pulmonary vasodilators for persistent pulmonary hypertension of the newborn: safety issues relating to drug administration and delivery devices. Medical Devices: Evidence and Research 2016;9:45-51. doi: 10.2147/mder.s99601
- Singh T, Nagaraja P, Bharathi K, Kaur P, Manjunatha N. Inhaled levosimendan versus intravenous levosimendan in patients with pulmonary hypertension undergoing mitral valve replacement. Annals of Cardiac Anesthesia 2018;21:328-332. doi:10.4103/aca.aca_19_18
- Zamanian R, Levine D, Bourge R, De Souza S, Rosenzweig E, Alnuaimat H, Burger C, Mathai S, Leedom N, DeAngelis K, Lim A, De Marco T. An observational study of inhaled-treprostinil respiratory-related safety in patients with pulmonary arterial hypertension. Pulmonary Circulation 2016;6(3):329-337 doi:10.1086/688059
- Anderson A, Dubosky M, Fiorino K, Quintana V, Kaplan C, Vines D. The effect of nebulizer position on aerosolized epoprostenol delivery in an adult lung model. Respiratory Care 2017;62(11):1387-1395 doi: https://doi.org/10.4187/respcare.05344
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.