How ECCO2-R works
Extracorporeal carbon dioxide removal (ECCO2-R) is a form of extracorporeal gas exchange designed to remove carbon dioxide from the blood across a gas exchange membrane at low blood flow rates (200-1500 ml/minute).1 This is done without a clinically relevant effect on oxygenation, as opposed to extracorporeal membrane oxygenation (ECMO), which is employed mainly for oxygen delivery at high blood flow rates (2,000-7,000 ml/minute). ECCO2-R has been referred to as low-flow ECMO and respiratory dialysis by some clinicians.2-3
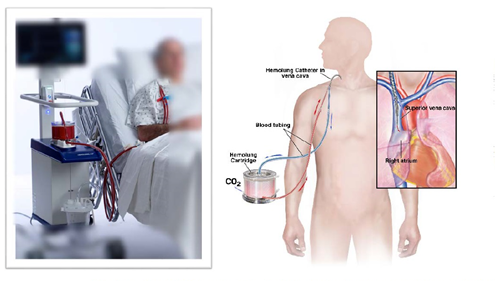
Figure 1. The Hemolung Respiratory Assist System. With permission from Alung Technologies, Inc.
ECCO2-R systems generally consist of a drainage cannula placed in a large central vein or artery, a gas exchange membrane, and a return cannula into the venous system. Figure 1 shows an ECCO2-R system called the Hemolung RAS from Alung Technologies, Inc. Some systems employ a double lumen cannula in which blood enters one lumen, then goes through the gas exchange membrane and back into the venous system through the second lumen contained in the same cannula (figure 2). Blood is pumped through the gas exchange membrane, where CO2 is removed by diffusion. A “sweep gas” that has little or no CO2 runs along the opposite side membrane, ensuring that there is a diffusion gradient that promotes CO2 removal. There are two general approaches: venovenous (VV)-ECCO2-R and arteriovenous (AV)-ECCO2-R.4
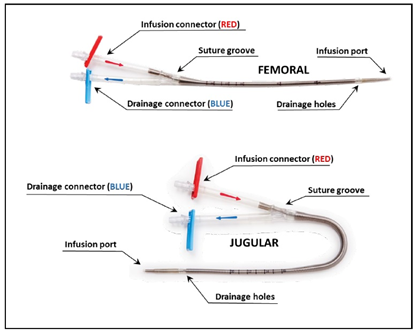
Figure 2. Double lumen catheters. With permission from Alung Technologies, Inc.
In the VV configuration, blood is drawn from a central vein through a drainage cannula using a centrifugal or roller pump to generate flow across the gas exchange membrane. The drainage cannula is conceptually the same as a dialysis catheter and hence is smaller than the typical ECMO catheter (13-19 Fr). CO2 is removed through diffusion to the sweep gas and returned to the venous circulation.
With AV-ECCO2-R, the blood flows from the femoral artery through a gas exchanger and back into the contralateral femoral vein. Sweep gas is again used to create the CO2 gradient allowing for diffusion. This type of system uses the patient’s cardiac output as the pump. AV-ECCO2-R needs an arteriovenous gradient > 60 mmHg and a cardiac index > 3L/min/m.2,5
The use of ECCO2-R is not without risk. The main complications associated with ECCO2-R are catheter-related complications (distal limb ischemia, compartment syndrome, and pseudoaneurysm formation), bleeding risk (despite the use of heparin-bonded circuits), hemolysis, and clot formation. Anticoagulation management can be a challenge and must be closely monitored.
Literature relating to ECCO2-R
While an entire literature review relating to ECCO2-R is out of the scope of this paper, areas of this subject where interest has increased will be reviewed. Experimentally, it has been shown that ECCO2-R devices can remove enough CO2 to allow a 50% reduction in alveolar minute ventilation leading to a significant reduction in PaCO2.3 Clinical evidence for the efficacy of CO2 removal dates to the late 1970s.6 The authors were able to show the CO2 removal by ECCO2-R would allow for lower frequency ventilation and lung rest.6 However, subsequent studies did not show positive outcomes, and the complication rates were relatively high.7-8
Current evidence supporting the use of ECCO2-R is limited. Most of the published data is observational and retrospective in nature. This introduces the possibility of selection bias, and results need to be viewed with this in mind. Most of the studies involve the use of ECCO2-R in patients with chronic obstructive pulmonary disease (COPD) and hypercapnic respiratory failure. There are also reports of ECCO2-R use for patients with ARDS, asthma exacerbations, during thoracic surgery, and as a bridge to lung transplantation.
Patients often require hospitalization and noninvasive ventilation (NIV) when they present with acute hypercapnic failure due to an exacerbation of COPD. This can potentially be a life-threatening situation, especially if the patient goes on to require endotracheal intubation and mechanical ventilation. ECCO2-R has appeal in this scenario to augment NIV and prevent invasive mechanical ventilation. Four small cohort studies were performed to examine the efficacy of using ECCO2-R to avoid invasive mechanical ventilation in patients at risk of NIV failure.9-12
All these studies were non-randomized, and none showed any definitive advantage in terms of intubation rate, length of stay, or mortality with the possible exception of Del Sorbo et al.11 Their results did point to a potential signal indicating a beneficial effect of ECCO2-R with respect to intubation rate and mortality. Currently, there are several studies ongoing examining the use of ECCO2-R in this patient population. Of note, because of its large randomized controlled nature (180 subject target), is the VENT-AVOID Trial (NCT03255057). The aim of this study is to examine the use of ECCO2-R in COPD patients experiencing an exacerbation who are: 1) At risk of NIV failure to try and avoid intubation and, 2) Patients who are intubated to try and enable earlier weaning. The study is ongoing at this time.
In patients with ARDS, ECCO2-R has appeal due to its ability to remove CO2 and help facilitate lung protective ventilation through a reduction in tidal volumes and plateau pressures. However, to date, there is only one prospective randomized controlled study comparing an ultra-protective ventilation strategy versus standard lung protective ventilation strategy in patients with moderate to severe ARDS.13 This study did show that ECCO2-R was workable and safe while, at the same time, enable the use of lower tidal volumes (3 ml/kg predicted body weight). Unfortunately, the study was stopped early since there were no differences in meaningful outcomes (duration of mechanical ventilation, length of stay, and mortality). However, a post hoc analysis revealed that there was a significantly higher proportion of successful weaning in subjects with severe ARDS (P/F ratio < 150).
Other areas where the ECCO2-R has generated interest include during thoracic surgery, as a bridge to transplant, and for asthma exacerbations. There are a few case reports of the ECCO2-R use in patients undergoing thoracic surgery to facilitate one-lung ventilation in patients who otherwise would not have tolerated the surgery.14 Patients awaiting lung transplantation who develop life-threatening hypercarbia may also benefit from ECCO2-R by using it as a bridge to transplantation. A couple of case series reports have reported the successful use of ECCO2-R in this clinical scenario.15-16 ECMO has been used in the past to manage asthmatics experiencing an exacerbation. However, if hypercarbia is the main problem, ECCO2-R may have more appeal because it is simpler to use and less invasive. A couple of case studies have reported its successful use.17-18
Role of the respiratory therapist
Due to its relative simplicity compared to ECMO, ECCO2-R can theoretically be used by any trained ICU staff. However, Respiratory Therapists (RTs) are most qualified to manage it. In all likelihood, patients in need of CO2 removal will be on either NIV or invasive mechanical ventilation, therapies primarily managed by RTs. An RT is usually one of the first to see hypercapnic patients in the emergency department, during rapid responses, and in the ICU for intubation.
Early identification of these patients is vital to prevent progression to invasive mechanical ventilation. After ECCO2-R is initiatied, CO2 removal becomes a balance between NIV/invasive mechanical ventilation management and ECCO2-R management (sweep gas in particular). Important parameters to monitor include respiratory rate, breathing pattern, arterial blood gases (ABG’s), and hemodynamic stability. ABG target values are generally: pH > 7.35, PaO2 > 55 mmHg, SpO2 > 88% FiO2 < 40%. Commencement of weaning from ECCO2-R is a clinical decision that is driven by the above-mentioned parameters. Once deemed ready to wean, the sweep gas is reduced until it is no longer needed – kind of a spontaneous breathing trial. If the patient’s respiratory rate, breathing pattern, hemodynamics, and ABG’s are stable, then the cannula can be removed at the decision of the clinical team.
Summary
ECCO2-R appears to be an effective therapy for patients experiencing hypercapnic respiratory failure and can enable clinicians to optimize lung protective ventilation. At the present time, however, the evidence is lacking that supports more widespread use, and ECCO2-R should be considered a research tool rather than an accepted clinical procedure. More robust research using randomized controlled trials targeting meaningful outcomes (intubation rate, duration of mechanical ventilation, length of stay, and mortality) is urgently needed.
References
- Schmidt M, Jaber S, Zogheib E, Godet T, Capellier G, Combes A. Feasibility and safety of low-flow extracorporeal CO2 removal managed with a renal replacement platform to enhance lung-protective ventilation of patients with mild-to-moderate ARDS. Crit Care 2018;22:122.
- Camporota L, Barrett N. Current applications for the use of extracorporeal carbon dioxide removal in critically ill patients. BioMed Res Int 2016;2016:Ide9781695.
- Batchinsky AI, Jordan BS, Regn D, Necsoiu C, Federspiel WJ, Morris MJ, Cancio LC. Respiratory dialysis: Reduction in dependence on mechanical ventilation by venovenous extracorporeal CO2 removal. Crit Care Med 2011;39(6):1382-1387.
- Morelli A, Del Sorbo L, Pesenti A, Ranieri VM, Fan E. Extracorporeal carbon dioxide removal (ECCO2R) in patients with acute respiratory failure. Intensive Care Med 2017;43(4):519-530.
- Cove ME, MacLaren G, Federspiel WJ, Kellum JA. Bench to bedside review: Extracorporeal carbon dioxide removal, past present and future. Crit Care 2012;16(5):232.
- Kolobow T, Gattinoni L, Tomlinson T, Pierce JE. An alternative to breathing. J Thoracic and Cardiovascular Surgery 1978;75(2):261-266.
- Gattinoni L, Kolobow T, Damia G, Agostoni A, Pesenti A. Extracorporeal carbon dioxide removal (ECCO2R): a new form of respiratory assistance. International J Artificial Organs 1979;2(4):183–185.
- Gattinoni L, Pesenti A, Mascheroni D, Marcolin R, Fumagalli,R Rossi F. Low-frequency positive-pressure ventilation with extracorporeal CO2 removal in severe acute respiratory failure. JAMA 1986;256(7):881–886.
- Kluge S, Braune SA, Engel M,Nierhaus A, Frings D, Ebelt H et al. Avoiding invasive mechanical ventilation by extracorporeal carbon dioxide removal in patients failing noninvasive ventilation. Intensive Care Med 2012;38(10):1632–1639.
- Burki NK, Mani RK, Herth FJF, Schmidt W, Teschler H, Bonin F et al. A novel extracorporeal CO2 removal system: results of a pilot study of hypercapnic respiratory failure in patients with COPD. Chest 2013;143(3):678–686.
- Del Sorbo L, Pisani L, Filippini C, Fanelli V, Fasano L, Terragni P et al. Extracorporeal CO2 removal in hypercapnic patients at risk of noninvasive ventilation failure: a matched cohort study with historical control. Crit Care Med 2015;43(1):120–127.
- Braune S, Sieweke A, Brettner F, Staudinger T, Joannidis M, Verbrugge S et al. The feasibility and safety of extracorporeal carbon dioxide removal to avoid intubation in patients with COPD unresponsive to noninvasive ventilation for acute hypercapnic respiratory failure (ECLAIR study): multicentre case-control study. Intensive Care Med 2016;42(9):1437–1444.
- Bein T, Weber-Carstens S, Goldmann A, Müller T, Staudinger T, Brederlau J et al. Lower tidal volume strategy (≈3 ml/kg) combined with extra-corporeal CO2 removal versus conventional” protective ventilation (6 ml/kg) in severe ARDS: the prospective randomized Xtravent-study. Intensive Care Med 2013;39(5):847–856.
- Wiebe K, Poeling J, Arlt M, Philipp A, Camboni D, Hofmann S, Schmid C. Thoracic surgical procedures supported by a pumpless interventional lung assist. Annals of Thoracic Surgery 2010;89(6):1782–1788.
- Schellongowski P, Riss K, Staudinger, T Ullrich R, Krenn CG, Sitzwohl C et al. Extracorporeal CO2 removal as bridge to lung transplantation in life-threatening hypercapnia. Transplant International 2015;28(3):297–304.
- Ricci D, Boffini M, Del Sorbo L, El Qarra S, Comoglio C, Ribezzo M et al. The use of CO2 removal devices in patients awaiting lung transplantation: an initial experience, Transplantation Proceedings 2010;42(4):1255–1258.
- Elliot SC, Paramasivam K, Oram J, Bodenham AR, Howell SJ, Mallick A et al. Pumpless extracorporeal carbon dioxide removal for life‑threatening asthma. Crit Care Med. 2007;35(3):945–948.
- Schneider TM, Bence T, Brettner F. “Awake” ECCO2R superseded intubation in a near‑fatal asthma attack. J Intensive Care 2017;5:53.
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.