Background
Knowledge regarding the exact causes, diagnosis, disease course, and ideal treatment for E-cigarette or Vaping use-Associated Lung Injury (EVALI/VALI) associated with e-cig/vaping (e-cig-V) remains largely unknown. Seeking to add outcomes to clarify information regarding these questions, Intermountain Healthcare critical care and pulmonary physicians conducted the largest EVALI/VALI study of its kind as of the manuscript publication date of December 2019.
Researchers further sought to determine if Telecritical Care (TCC) could effectively be used as a central repository to diagnose, treat, track symptoms, and guide the best clinical care of this subset of subjects.
This multi-site, prospective, observational cohort study included EVALI subject tracking from June 27-October 4, 2019. Researchers extracted data regarding clinical presentation, treatment, and two-week follow-up post-hospital discharge prospectively from electronic medical records and subject interviews.
Sixty subjects presented at 13 hospitals with lung injury and a history of e-cig-V. Of these, 33 (55%) were admitted to an intensive care unit (ICU). Fifty-nine (98%) presented with respiratory symptoms, 53 (88%) with constitutional symptoms such as fever and chills, and 54 (90%) with gastrointestinal symptoms. Fifty-four (90%) were treated with antibiotics and 57 (95%) with steroids. Six (10%) subjects were readmitted to the hospital within two weeks of discharge. Of these six, 3 (50%) relapsed with e-cigarette/vaping use. Of the 26 subjects with follow-ups within two weeks, 10 of 15 (67%) had continued abnormalities on chest x-rays, and 6 of 9 (67%) had abnormal pulmonary function tests. Two subjects died. The lung injury due to e-cig-V use was thought to be a contributing cause; however, it was not the direct cause of death.
Severe lung injury, constitutional and gastrointestinal symptoms are associated with e-cig-V use. Though some improvement was noted in the short-term with the use of antibiotics and steroids, many subjects had continued abnormalities on a two-week follow-up. There is a need to understand the pathologic causes, optimal therapy, and longer-term outcomes for this emerging disease.
Current knowledge
Early recognition of emerging diseases using a standardized approach for identification, communication, treatment, and short-/long-term tracking and reporting of data outcomes is lacking. This deficiency is especially true in the under-researched area of e-cigarette/vaping impact on public health.
What this study contributes to our knowledge
This study reports the use of TeleCritical Care as a central repository for outcomes and standardizing care for the largest study regarding lung injury induced by e-cigarette/vaping as of 2019. In addition, it introduces a guideline for history capture, signs, symptoms, differential diagnosis, subject disposition, treatment, and follow-up. These findings are helping all clinicians in recognizing what lung injuries associated with e-cigarettes or vaping look like and helping them maintain a high degree of suspicion for diagnosing and treating lung injuries in subjects who have been vaping.
Diving into the study
One of the first acknowledgments of the national public health concern that e-cig-V was linked to an emerging outbreak of lung injury among adults was published by the U.S. Surgeon General in 2016.2 National e-cig-V prevalence, as reported in 2018, estimated 8.1 million U.S. adults were current e-cig users.1 The 2018 National Health Data Survey reported three key findings to include: 1) 14.9% of adults had ever used an e-cig, and 3.2% were current users, 2) the prevalence of adults who had ever used an e-cig and were current users was highest among men, non-Hispanic white adults 18–24 years of age, and 3) adults who quit smoking cigarettes within the past year were found to be the most likely to have ever used (57.3%) and to be current (25.2%) e-cig users.2
Details regarding e-cigs-V remain unknown in terms of the exact cause(s) of lung injury, knowledge regarding the clinical presentation, optimal treatment, and short/long-term sequala. There is no current test for this disease. Ingested Lipid-laden macrophages have been identified with bronchoalveolar lavage samples; however, neither the clinical course, radiographs, or pathological sampling support lipid pneumonia as the mechanism of the lung injury.3 With the 2018 outbreak and severity of cases, the researchers suspected perhaps a change in the e-liquid composition in the US, and specifically in Utah, may have been a contributing factor.
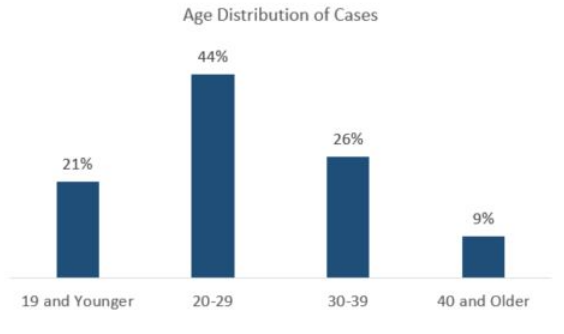
Several initiatives for Utah EVALI tracking were being conducted during 2018 – 2020. In 2019-2020, EVALI cases were reported to the Utah Department of Health by clinicians and the Utah Poison Control Center. EVALI prevalence was reported as 26 cases/1,000,000 population. The number of EVALI in Utah is among the highest in the nation.4,5 A final Lung Injury Outbreak Data report from the Utah Department of Health (UDOH) continued to track EVALI cases from April 2019 – January 2020, which followed the initial outbreak identified in 2018.6 EVALI case counts during that time frame were 134. Most cases were subjects in their 20s and 30s with a median age of 26. The age distribution is reported in Figure One. Gender distribution was 72% male and 28% female. Subjects self-reported vape liquid content use as 89% with tetrahydrocannabinol (THC) and 60% with nicotine. The UDOH’s Utah Public Health Laboratory worked with state and national laboratories to test products used by case subjects. Thirty-nine samples were tested for chemical compounds that included illicit drugs such as opioids, fentanyl, methamphetamines, cutting agents, and other biologic toxins. Of the 39 products tested, 19 (49%) were THC cartridges, and 20 (51%) were nicotine e- juices. Almost all THC cartridges tested positive for vitamin E acetate.
Figure One: UDOH’s EVALI Age Distribution: Final Lung Injury Outbreak Report from April 2019 – January 20206
In contrast, no nicotine products tested showed any unexpected compounds. Vitamin E acetate is common in several consumer products and used as a diluent for THC-containing vape products. It helps with the appearance and taste. It also serves as a filler of sorts to lower the cost of producing the vaping liquid.7 One theory regarding the impact of inhaled Vitamin E acetate is that surfactant, which reduces surface tension at the air-liquid interface and provides stability against lung collapse, may be disrupted due to the oil in Vitamin E acetate.8 Davidson et al. proposed another theory that aerosolized oils deposit in distal airways and alveoli causing a local inflammatory response. This oil deposit impairs gas exchange. Thus, they conclude symptoms of lipoid pneumonia are often nonspecific with variable chest imaging, which can lead to a delayed or missed diagnosis.9 Most of the patients with vaping-related lung illness included in the Intermountain Healthcare initial study had vaped unregulated THC cartridges, allowing the user to consume cannabis oil via vaping.
Recognizing EVALI an emerging illness in Utah during 2018, physicians within Intermountain tracked cases as they were presented. Intermountain Healthcare is an integrated health care organization serving Utah and parts of Idaho, Wyoming, and Nevada, with 24 hospitals and approximately 160 clinics. A group of the organization’s physicians conducted the largest study of its kind regarding EVALI with outcomes reported December 2019 in Lancet.10 These innovative researchers clarified two key study objectives. First, they sought to gather data to add to the growing body of literature regarding the EVALI clinical course, treatment, and short-term outcomes of this subset of patients. Secondly, they sought to determine what, if any, impact the use of Telecritical Care (TCC) as a central repository to assist with rapid identification of a public outbreak, establishment of a specialized task force, rapid dissemination of knowledge, and the utilization of a proposed guideline for the evaluation and treatment of EVALI. A synopsis of their study is reported here as well as the reporting of implications for Respiratory Therapists.
Study Design
This multi-site, prospective, observational cohort study included data collection on all subjects with lung injury associated with e-cig-V seen at an Intermountain Healthcare facility between June 27-October 4, 2019. Researchers used a previously published case series of 53 subjects where severe acute lung injury was defined in those with vaping exposure within 90 days prior to the patient seeking medical treatment for symptoms as a reference for this study.11 The case definition for the diagnosis of EVALI required: 1) the use of e-cigarettes within 90 days of symptom onset, 2) pulmonary infiltrates on chest radiograph or chest CT, and 3) an absence of other known causes, such as infection.
The Protocol
The Institutional Review Board approved the Intermountain Healthcare study at Intermountain Healthcare with subject consent waived. The study was funded by the National Heart, Lung and Blood Institute and the Intermountain Research and Medical Foundation. A guideline was created to standardize the approach of collecting vape history, acute lung injury exposure history, signs, symptoms, differential diagnosis, EVALI case study definition, disposition based on clinical presentation and post-hospital discharge follow-up (Figure Two).10
Figure Two: Intermountain Healthcare’s Proposed EVALI Patient Assessment and Treatment Guideline in First Study9
Study Guideline Page 1 and Page 2
Note: Physician researchers acknowledged Respiratory Therapists for their contributions to the guideline in the publication.
Subjects
Subjects were identified as meeting the criteria during routine care at all points of patient care to include hospital admission, emergency department, and/or outpatient clinic visits as detailed in Figure Three.10 Subject demographic data are presented in Figure Four.10
Figure Three: Intermountain Healthcare’s First Study: Screening, Admittance, and Treatment of Patients with Lung Injury Associated with E-Cigarette/Vaping Use9
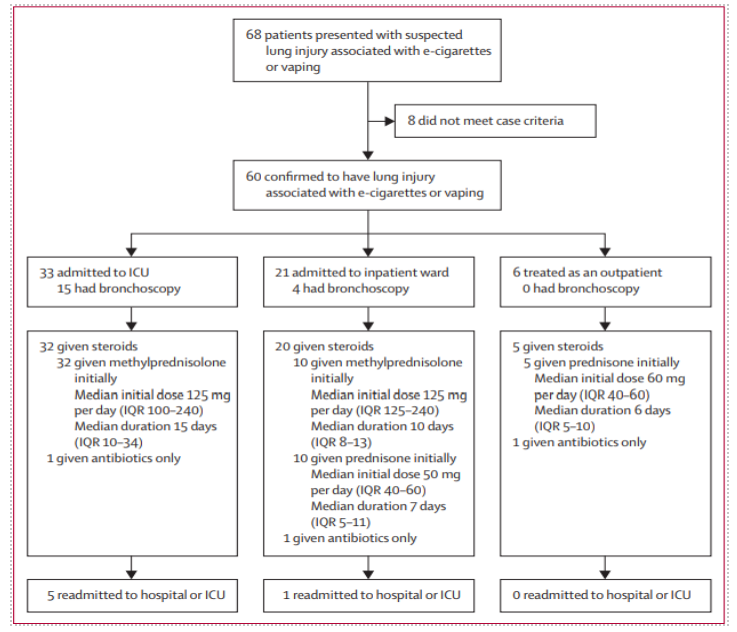
Figure Four: Intermountain Healthcare’s Subject Demographics of First Intermountain Healthcare Study9
Intervention
A task force of five pulmonary and critical care physicians was used to review the case index for inclusion following the study guideline. Researchers documented prospectively subject clinical presentation, treatment, and two-week follow-up post-hospital discharge as well as data extracted via patient electronic medical records and subject interviews. Where an agreement for inclusion was questioned, more than one physician was consulted. Designated members of the TCC team performed interviews.
A system email was created and distributed to all clinicians regarding referral requests when criteria were felt to be met. Included study subjects were also reported to the Utah Department of Health, who also performed a detailed review. TCC support was used for all subjects with ICU admission and others as requested.
Treatment
The majority of subjects received antibiotics, steroids, and supplemental oxygen (Figure Three). The physicians’ impression was the most rapid improvement was due to the steroids. However, they stopped short of suggesting steroid use as a best practice, citing more investigation.
Statistical Analysis
Due to the study’s exploratory nature, researchers reported the presence of signs and symptoms, clinical course, treatment, and outcomes in descriptive statistics and InterQuartile Ranges (IQR). Thus, researchers explained no p-values were reported.
Results
Sixty-eight subjects were initially referred to the study. Eight subjects did not fit the case definition and were excluded leaving a total of 60 for study inclusion. A total of 71 cases of EVALI were reported in Utah during the described time frame. Of these, 60 (85%) were identified and care for at an Intermountain Healthcare hospital or outpatient clinic. The first patient identified with EVALI was seen on June 27, 2019, before realizing that an outbreak was emerging. Thirty-five (58%) presented to tertiary hospitals, and 17 (28%) were transferred from smaller facilities. Eight (32%) were able to be cared for at the smaller hospitals they initially presented.
Researchers reported almost all subjects presented with respiratory, constitutional, and gastrointestinal complaints. Respiratory presentation included oxygen saturation < 88%, respiratory rate > 20/minute and tachycardia > 100 beats/minute. Figure Five is an example of a chest radiograph of a subject in the initial Intermountain Healthcare study depicting the severe inflammation resulting from e-cig-V use. Thirty-three (55%) subjects were admitted to the ICU, with 10 (17%) requiring mechanical ventilation. Lab findings included mild increases in liver function tests, and white blood cell counts greater than 11000/mm3. Inflammatory markers were often high (i.e., median C-reactive protein concentration 31 mg/L [IQR 21-33] and median erythrocyte sed rate of 92 mm/h [IQR 65-103]). Two (3%) subjects had elevated creatinine concentrations higher than 2 mg/dL. The severity of illness varied from those requiring ICU admission and mechanical ventilation to 6 (10%) being cared for in the outpatient setting. No patients had a microbiology result that would indicate infectious pneumonia. As reported in previous studies, 8 (89%) of 9 bronchoscopy samples reported lipid-laden macrophages.3
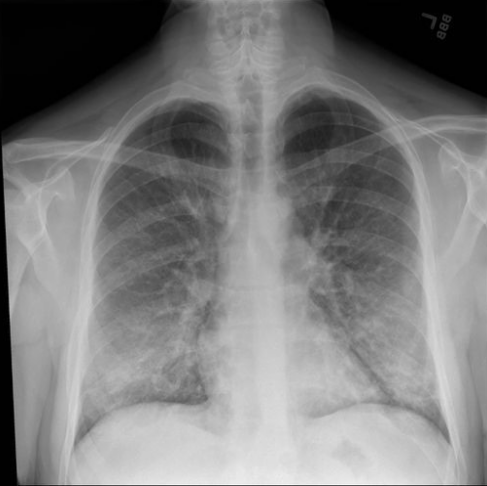 Photo courtesy of Intermountain Healthcare. An x-ray of the lungs released as part of the initial Intermountain Healthcare study showing severe inflammation in a subject with e-cig-V history and illness.
Photo courtesy of Intermountain Healthcare. An x-ray of the lungs released as part of the initial Intermountain Healthcare study showing severe inflammation in a subject with e-cig-V history and illness.A key finding was the incidence of pneumothorax and pneumomediastinum noted in 11 (18%) of the total subjects studied. One patient had a pneumothorax and pneumomediastinum, one had a pneumatocele that eventually became infected, and one was diagnosed with emphysematous gastritis. Emphysematous gastritis in conjunction with e-cig-V use has been previously reported in the literature.12 Emphysematous gastritis occurs when gas-producing organisms cause a mucosal defect in the gastric wall, causing redness and swelling of the stomach lining resulting in pain reported by subjects. This condition can be a lethal infection. It most often is associated with excessive alcohol consumption, eating spicy food, and smoking.
Vape liquid was predominantly purchased via online, social media, or a friend, with only one patient admitting to making their own. The longest reported user was five years, with the median use being 225 days (IQR 90-620).
Discussion
Intermountain Healthcare researchers have contributed significantly to the accumulating data regarding the early identification, treatment, and residual issues of EVALI subjects. Key findings include rapid outbreak identification, the use of a proposed standardization guideline, the formation of a specialized task force, and the centralization of data and reporting using TCC. These led to improved, standardized recognition, diagnosing, and treatment of subjects with e-cig-V induced lung injury. The researchers reported an increased ability to recognize, treat and follow EVALI patients. They further suggest the use of a central repository such as TCC may be of benefit in other emerging public health issues. Finally, the authors suggested public awareness that flu-like symptoms and abdominal symptoms in conjunction with e-cig-V use within 90-days of symptom presentation, even in the absence of respiratory symptoms, may be early signs of e-cig-V associated health compromise.
Table One: Intermountain Healthcare Second Study of EVALI Outcomes in 111 Subjects Followed Thirty Days Post-Hospital Discharge Between June 1, 2019 through January 13, 2020 9
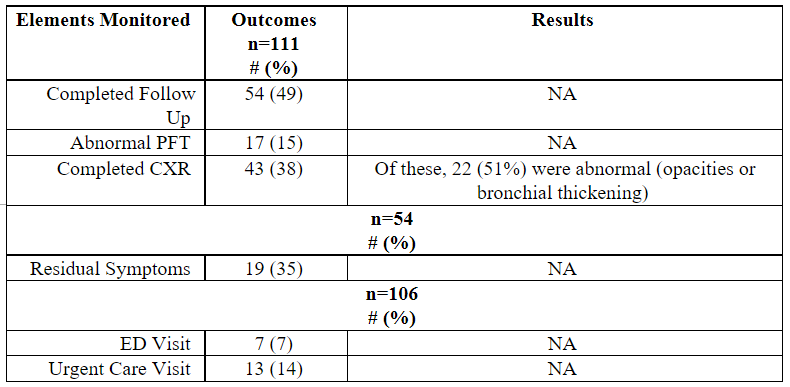
Note: 114 subjects were identified; however, 1 died before discharge and 2 died within 30 days of hospital discharge, leaving 111 subjects for inclusion.
Blagev et al. performed a subsequent study reported as a poster presentation in 2020 at the American Thoracic Society’s International Conference.13 They followed another 114 subjects in a second study diagnosed with EVALI reviewing short-term follow-up outcomes for pulmonary function testing, chest radiographs, symptoms, and 30-day hospital readmissions. Outcomes are reported in Table One. In terms of hospital readmissions within 30 days, they reported a statistically significant association between more prolonged initial hospital admissions and an increased chance for readmission within 30 days (p=0.013). In addition, once readmitted, subjects had a more extended hospital stay (median 10 vs. 4 days, p=0.0045) but no longer ICU stays (median 0 vs. 1.5 days, p=0.18).
In an interview with Fox News, Denitza Blagev, MD, stated: “EVALI is a serious disease that has serious complications from the start. Now, we’re finding the long-term consequences are akin to those who have had sepsis or acute respiratory distress syndrome. These patients ‘recover,’ but even in that recovery, our study shows that for a percentage of those patients, they may never return to normal.” Dr. Blagev is a pulmonary and critical care physician at Intermountain Healthcare and the study’s principal investigator.
Conclusion
One might argue that more questions arose than were answered by the initial study and the subsequent study; however, both Intermountain Healthcare studies contribute significantly to the growing body of evidence regarding EVALI. With about a quarter of the patients diagnosed with EVALI having a previous diagnosis of asthma, a large volume of questions remains unanswered. Questions include 1) Does e-cig-V use increase the likelihood of developing lung injury where asthma is a comorbidity? 2) Are there biological differences between female and male propensity to develop EVALI since more men than women in the study were diagnosed? This predominance of males was also noted in the UDOH’s Final Lung Injury Outbreak Report in January 2020. 3) What explains the relatively high occurrence of pulmonary air leaks in patients diagnosed with EVALI? Is it the oil disruption of the surfactant production and thus increased surface tension that is the culprit? 4) Should routine public health/hospital laboratory testing for e-cig-V liquids be performed to monitor additives when patients present to hospitals with suspected symptoms of EVALI, thus enabling more rapid response and analysis of potential causes of future emerging EVALI cases? While the FDA has regulatory authority over vaping and does examine the contents of the major brands of e cigs, there are still unregulated, home grown manufactures whose vape juice content is unknown. What, if any, short- and long-term sequela persist after e-cig-V cessation, and is this permanent or reversible?
Respiratory therapists, respiratory care students, and other key health organizations lobbied during Utah 2014, 2015, and 2016 open legislative sessions for e-cig-V regulation in manufacturing, marketing, and distribution of e-cig-V products. Legislators responded with a request for more data. That challenge has been heard by Intermountain Healthcare researchers, the UDOH, and others across the nation. However, research still begs to be completed to address the questions raised in this paper and to fulfill legislative requests for more evidence. Respiratory therapists have a vital role in the care of subjects experiencing respiratory distress due to e-cig vaping. The findings of this study should be considered in subjects meeting the case study definition of EVALI in terms of clinical presentation (respiratory, gastrointestinal, and constitutional symptoms) with a history of e-cig-V use in the 90 days and previously diagnosed with asthma. Respiratory Therapists are uniquely qualified to research this area. Teaming with other assisting agencies such as local health departments may help with funding, resources, and assist in the development of community partnerships. Doing so advances our profession as innovative professionals who identify best practices. The challenge is ours for the taking.
References
- National Center for Chronic Disease Prevention and Health Promotion. E-cigarette use among youth and young adults. A report of the Surgeon General (2016). Retrieved from: https://e-cigarettes.surgeongeneral.gov/documents/2016_SGR_Full_Report_non-508.pdf (accessed August 4, 2021).
- Creamer MR, Ang TW, Babb S, Cullen KA, Day H, Willis G, et al. Tobacco product use and cessation indicators among adults-United States. MMWR Mob Mortal Wkly Rep 2018; 68(45):1013-1019. Retrieved from: http://dx.doi.org/10.15585/mmwr.mm6845a2 (accessed August 3 2021).
- Maddock SD, Cirulis MM, Callahan SJ, et al. Pulmonary lipid-laden macrophages and vaping. N Engl J Med 2019; 381;1488-1489.
- Lewis N, McCaffrey K, Sage K, et al. (2019). E-cigarette use, or vaping, practice and characteristics among persons with associated lung injury. MMWR Morb Mortal Wkly Rep 2019; 68:953-956.
- United States Census Bureau. Quickfacts: Utah. US Department of Commerce (2018). Retrieved from: https://census.gov/quickfacts/fact/table/UT/PST045218 (accessed August 3, 2021).
- Utah Department of Health. Lung injury outcomes data (2020). Retrieved from: https://health.utah.gov/lung-disease-investigation/lung-injury-outbreak-data (accessed August 2, 2021).
- Kamal MA and Raghunathan VA. Modulated phases of phospholipid bilayers induced by tocopherols. Biochim Biophys Acta. 2012;1818:2486–2493.
- Boudi FB, Patel S, Boudi A, and Chan C. Vitamin E Acetate as a Plausible Cause of Acute Vaping-related Illness. Cureus 2019;11(12), e6350. https://doi.org/10.7759/cureus.6350
- Davidson K, Brancato A, Heetderks P, et al. Outbreak of electronic-cigarette associated acute lipoid pneumonia—North Carolina, July-August 2019. MMWR Morb Mortal Wkly Rep 2019;68:784-786 DOI: http://dx.doi.org/10.15585/mmwr.mm6836el.
- Blagev DP, Harris D, Dunn AC, Guidry DW, Grissom CK and Lanspa MJ. Clinical presentation, treatment, and short-term outcomes of lung injury associated with e-cigarettes or vaping: a prospective observational cohort study. Lancet 2019; 394:2073-2083.
- Layden JE, Ghinai I, Pray I, et al. Pulmonary illness related to e-cigarette use in Illinois and Wisconsin-preliminary report. N Engl J Med 2019; published online September 6. DOI:10.1056/NEJMoa1911614.
- Wong YY and Chu WC. Emphysematous gastritis associated with gastric infarction in a patient with adult polycystic renal disease: CT diagnosis. AJR AM J Roentgenol 2002; 178:1291.
- Blagev DP, Harris D, Anderson B, Callahan SJ, Rea S, Guidry DW, Grissom CK, Lanspa MJ. Short Term Follow-Up and Readmission Risk Factors in Patients with E-Cigarette, or Vaping, Associated Lung Injury (EVALI). ATS 2020 International Conference (2020). Retrieved from: https://abstractsonline.com/pp8/#!/8998/presentation/17575 (accessed August 3, 2021).
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.