Without breath, there is no life! Therefore, respiratory therapists are among the first healthcare providers (HCPs) at the bedside during emergency health situations, in which aerosol-generating procedures (AGPs) may be needed. Since the highly contagious SARS-CoV-2 increased concerns on viral transmission via aerosols generated by medical procedures, it is vital to bridge the gap between treating patients with COVID-19 effectively and protecting HCPs from the viral transmission.
According to previous research, the virus is shed as a wide range of particles,1,2 and factors such as closer proximity, long exposure, and forced exhalation of air from a patient with a high viral load may lead to cross-infection with COVID-19.1 Wearing personal protective equipment (PPE), performing AGPs in negative pressure rooms, and adhering to strict infection control and prevention guidelines are logical implications for managing patients with COVID-19. Although our knowledge and understanding of the virus and the treatment of COVID-19 continue to evolve, the purpose of this paper is to evaluate the relevance of each AGP and provide some pragmatic strategies to clinicians about how to use AGPs safely and effectively during this pandemic.
Aerosol Therapy
The COVID-19 pandemic raised concerns about the use of aerosolized medications and viral transmission through infected aerosols. Therefore, it is vital to differentiate medical aerosols from bioaerosols. Medical aerosols are produced by nebulizers or inhalers and are not the same as bioaerosols generated by patients during talk, cough, sneeze, or sing. Bioaerosols will carry the pathogen if the patient is infected by COVID-19.3,4,5 That may not be the case with medical aerosols unless the aerosol device or the medication gets contaminated.3,4,5 Also, the medical aerosol coming in contact with an infected mucous membrane in the lung stops being airborne and is no longer considered as an aerosol.6
Due to less aerosol generation, lower emitted dose, shorter treatment time, and the medication that is contained in the device, inhalers have less risk for device contamination than nebulizers and are preferred in spontaneously breathing patients with COVID-19.3,4,5 Pressurized metered-dose inhalers (pMDIs) should be used with a valved holding chamber to minimize the need for hand-breath coordination, decrease oropharyngeal deposition, and increase lung dose.3,4,5 Dry powder inhalers (DPI) should be used in patients who can generate high inspiratory flow rates needed to draw the drug from the DPI and disperse drug particles within the respirable range during therapy.3,4,5 However, high inspiratory flow rates needed for the effective use of DPIs may lead to cough and airway irritation that will increase the dispersion of exhaled bioaerosols, and viral transmission to the environment.3,4,5 Inhalers producing exhaled bioaerosols due to cough or airway irritation are not superior to aerosol drug delivery with nebulizers.
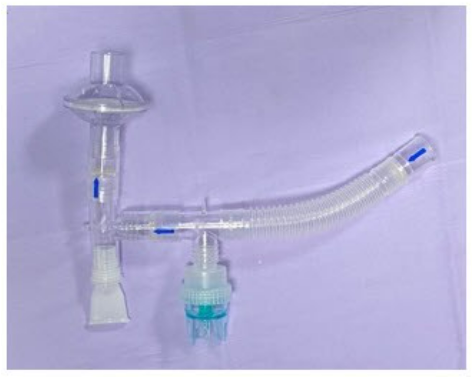 Image 1. Jet nebulizer with one-way exhalation valves and filter.
Image 1. Jet nebulizer with one-way exhalation valves and filter.Whereas the maximum dispersion distance of exhaled aerosols was greater than 0.45 m with the conventional jet nebulizers attached to a face mask,7 aerosols generated by a nebulizer may not contain the pathogen unless the device is contaminated. Tang et al8 used a live attenuated influenza vaccine as a surrogate virus tracer to collect air samples in three different locations after aerosol therapy with a jet nebulizer attached to a face mask. They reported that air samples included 612 viruses/L near the head, 174 viruses near the abdomen, and 118 viruses/L near the feet in an isolation room with 12 air changes per hour. Attaching a filter to the exhalation port of the nebulizer (Image 1) and using a mouthpiece instead of a face mask significantly reduces the mass concentration of aerosols in the environment.9 Although an aerosol mask with viral/bacterial filters may be used during this pandemic, the efficiency of filtered masks in reducing aerosol dispersion to the environment and viral transmission remains to be elucidated by clinical trials. Also, it is essential to utilize an aseptic technique during nebulizer preparation, cleaning, and maintenance to avoid nebulizer contamination in the era of COVID-19 and beyond.
The type and design of the nebulizer play an essential role in the risk of device contamination and viral transmission.4 For instance, conventional jet nebulizers have an open design and are placed below the gas pathway as opposed to mesh nebulizers separating the medication from the interface through the mesh. Conventional jet nebulizers have large residual volumes (~2 mL) that may create a hospitable environment for pathogens if the nebulizer is not cleaned after each treatment. They also operate with an external gas flow that increases the dispersion of exhaled aerosols to the environment.4 Mesh nebulizers are good alternatives to jet nebulizers because they have low residual volumes and operate with electrical mains or batteries. Another option to conventional jet nebulizers is to use breath-actuated nebulizers in spontaneously breathing patients with COVID-19.4
Device selection in the era of COVID-19 should be tailored specifically to patients’ abilities and clinical conditions.4 For instance, inhalers should be preferred in spontaneously breathing patients, while nebulizers should be used in patients who cannot perform the optimum delivery technique with inhalers, when drug formulation is not available as an inhaler, and in patients receiving mechanical ventilation, noninvasive ventilation (NIV) and high flow nasal cannula therapy.10 When nebulizers are used in these patient populations, clinicians should utilize the suggestions listed in Table 1 to provide safe and effective delivery of aerosolized medications to patients with COVID-19. For instance, using a dual limb ventilator circuit and keeping the circuit of the ventilator intact is vital to avoid viral transmission during NIV. Since breaking the circuit for the placement of the pMDI is a risk for exposure, HCPs should prefer using a jet nebulizer with a valved-T piece or a mesh nebulizer during NIV and place a HEPA filter to the expiratory limb of the circuit to minimize the dispersion of exhaled aerosols to the atmosphere. In ventilator-dependent patients, it is essential to keep the ventilator circuit intact to avoid viral transmission.4,5 Therefore, mesh nebulizers or jet nebulizers with a valved T-piece should be used for aerosol drug delivery to ventilator-dependent patients. Also, clinicians should attach a high efficiency particulate air (HEPA) filter to the expiratory limb of the ventilator to reduce secondhand aerosol exposure and viral transmission during mechanical ventilation.4,5,11 If the patient needs continuous nebulization, clinicians should use the in-line placement of a nebulizer with the HFNC set-up. In this case, the nebulizer should be positioned before the humidifier to improve the delivery efficiency of the treatment.10 Also, a surgical mask should be placed on the patient’s face to prevent the transmission of infectious bioaerosols during HFNC therapy.10
Oxygen Therapy
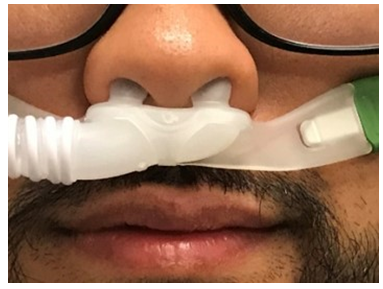 Image 2. Snug-fitted HFNC.
Image 2. Snug-fitted HFNC.Supplemental oxygen is a vital treatment for patients with hypoxemic respiratory failure. The COVID-19 pandemic has raised many questions regarding the role of oxygen therapy devices and the dispersion of bioaerosols. An in vitro study by Hui et al. evaluated exhaled air dispersion during nasal cannula usage, which showed an increase in exhaled air dispersion as the oxygen flow was increased from 1 to 5 L/min with substantial exposure 1 m from the bed in a negative pressure room.12 The same study showed that air entrainment masks further increased exhaled air dispersion when compared with simple masks and nonrebreather masks. Based on this study, it is recommended that a surgical mask be placed on patients receiving oxygen therapy via standard nasal cannula (Table 1). It is also recommended to avoid using Venturi and nonrebreathing masks unless they are filtered.12
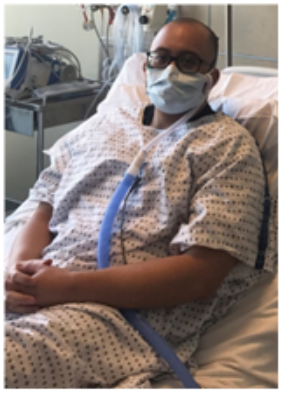 Image 3. HFNC with surgical mask.
Image 3. HFNC with surgical mask.In tracheotomized patients, clinicians should prefer using the T-piece over the tracheostomy mask for oxygen therapy and attach a filter to the exhalation port of the circuit to reduce the dispersion of exhaled aerosols to the atmosphere. It is also essential to frequently check and replace the filters when necessary (Table 1).
High Flow Nasal Cannula
High flow nasal cannula (HFNC) is widely used for patients with hypoxemic respiratory failure. The ability of the high flow oxygen therapy to meet high inspiratory flow demands, reduce oxygen dilution, and washout pharyngeal dead space has led to improved patient comfort and reduced the need for endotracheal intubations when compared to other standard oxygen delivery devices.12 The use of HFNC has been a topic of discussion due to high flow rates up to 60 L/min and the potential to generate and disperse bioaerosols. A study by Bem et al.13 visualized, and quantified particles and droplets generated by healthy patients using a nonrebreather mask or HFNC. The study noted no difference in aerosolized particles and failed to detect increased dispersion of bacteria and viruses between HFNC and nonrebreather masks.13
The HFNC is considered safe with a low risk of exposure to aerosolized particles for HCPs in the setting of an airborne infection isolation room (AIIR) or well-ventilated room when an AIIR is not available.14 Current recommendations based on the evidence supporting the efficacy and safety of HFNC recommend patients use a snug-fitting cannula with a surgical mask (Image 2 and 3), while HCPs are in the room to help limit the potential for droplet dispersion during therapy (Table 2).12
Non‐invasive Ventilation
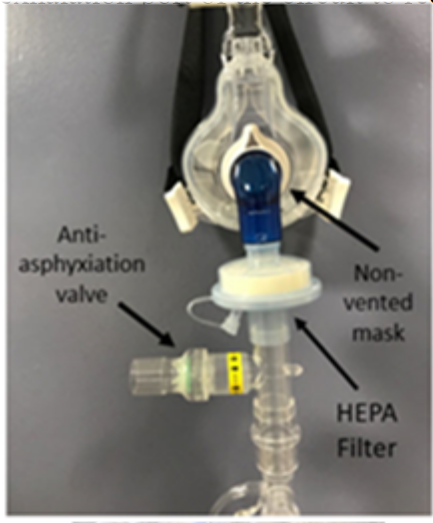 Image 4. Non-vented NIV mask with HEPA filter attached to single lim b circuit.
Image 4. Non-vented NIV mask with HEPA filter attached to single lim b circuit.NIV is often considered as a bridge to mechanical ventilation and has been shown to reduce the intubation rate and 90-day mortality rate in patients with mild to moderate ARDS.12 The presence of leaks from exhalation ports and patient interfaces increases the dispersion of bioaerosols and puts HCPs at an increased risk for exposure. Hui et al.3 conducted an experimental study using a breathing simulator, which reported an exhaled air dispersion within a 0.5 m radius that increased with higher pressure settings. HCPs that are in close proximity to patients receiving NIV should wear appropriate PPE for AGPs as well as an N95 or appropriately sized air-purifying respirator. To reduce the dispersion of bioaerosols from the patient, a dual limb circuit with a non-vented mask (Image 4) and a HEPA filter placed at the expiratory port of the ventilator is recommended (Table 2). The patient should be fitted with an oronasal mask. If the oronasal mask does not produce a good seal, a total face mask or helmet can be used. Nasal masks should be avoided. Also, limiting the leak from the circuit and patient interface is crucial to reducing bioaerosol dispersion.12
As shown in Image 4, when using a single limb circuit, a filter should be placed before the exhalation port (Table 2). Humidification should be avoided due to the risk of the viral filter capturing droplets and preventing exhalation. The use of single-limb non-invasive circuits is not recommended in non-negative pressure rooms. If humidification is necessary, a dual limb ventilator circuit and non-vented mask can be used with filters placed at the expiratory port of the ventilator to reduce exhaled gas and aerosol dispersion.12
Manual Ventilation and Intubation
While noninvasive respiratory care management strategies can be successfully used to treat acute hypoxemic respiratory failure, it is prudent to monitor respiratory drive and effort to identify worsening respiratory failure and to avoid delayed endotracheal intubation. Several studies have reported an association between AGPs and HCP infection during the SARS CoV-1 epidemic.15-23 All of the studies are retrospective cohort and case-series, none of which measured aerosol levels. Tran et al.24 conducted a systematic review of the literature to identify the risk of transmission acute respiratory infections when comparing HCPs who were either exposed to AGPs or not. Tracheal intubation was identified as an independent risk factor for SARS transmission to HCPs, and the odds of SARS transmission-related risk were significantly high with manual ventilation as well. Prolonged exposure, poor infection control measures, and inadequate compliance with infection control and prevention guidelines may have contributed to nosocomial transmission.
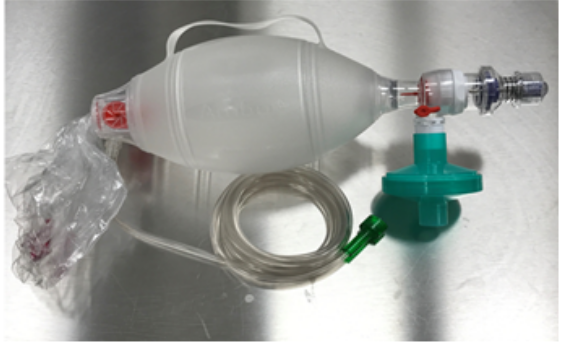 Image 5. HEPA filter connected to manual resuscitation bag.
Image 5. HEPA filter connected to manual resuscitation bag.The Center for Disease Control (CDC), World Health Organization (WHO), and several U.S. health organizations have identified tracheal intubation as an AGP. In preparation for tracheal intubation, infection control recommendations to reduce the risk of infection transmission includes the use of N95 or higher level respirator, eye protection, gown, double gloves, and placement of the patient in an AIIR or well-ventilated room when an AIIR is not available.25,26 Chan et al27 used a human patient simulator to evaluate the extent and direction of exhaled air dispersion during bag-mask ventilation (BMV) by HCPs with varying levels of experience. Twenty participants, inclusive of medical students, anesthesiologists, pulmonologists, and non-ICU clinic nurses, were allowed to practice BMV for up to 30-minutes using three different manual ventilation devices, one of which had a mechanical filter placed between the resuscitation device and mask. Participants were instructed to deliver 10-12 breaths per minute for 3-minutes while smoke particles of less than 1 µ were produced by a smoke generator; the smoke leakage jet plume was illuminated by laser light. Smoke plume particle concentration was then measured, and images were generated to visualize the pattern of exhaled air dispersion as an indicator of the probability of coming into contact with potentially infectious exhaled air. Transverse plane dispersion distance ranged from 5-inches to 11-inches, which is the typical distance separating a clinician performing direct laryngoscopy from the patient’s airway during tracheal intubation. Group comparisons identified that clinic nurses produced significantly larger leaks (9-inches to 11-inches) when compared to the other participants (P < 0.001). Adding a filter between the manual resuscitation device and mask resulted in less leakage when BMV was performed by the anesthesiologists, pulmonologists, and medical students who had more experience with the procedure when compared to the nurses. This study supports the recommendation for adding a HEPA filter to a manual resuscitation device whenever it is used (Image 5).12,28
Since HCPs who perform or assist with endotracheal intubation come into close proximity to the patient’s airway, this procedure is directly associated with a high risk of viral pathogen exposure and transmission. To reduce the risk of exposure, only essential HCPs should be in the room during intubation. Proper PPE, including double gloving, is advised. Rapid sequence induction and intubation should be performed by the most experienced clinician because they are most likely to achieve a rapid and greater chance of first-time intubation attempt success when compared to a less experienced intubator.29,30 In anticipation that hypoxemia may occur during intubation, pre-oxygenation with 100% oxygen and an oxygen delivery device other than BMV, such as HFNC or non-rebreather mask, is recommended.12,30 Proximity to the airway and the amount of time in close proximity to the airway are an important factor related to viral exposure and possible transmission. Rapid sequence induction and intubation with the use of disposable (single use) laryngoscope, which the incubator finds most comfortable and likely to achieve rapid intubation, is paramount. A videolaryngoscope with a disposable cover is recommended if available because it creates a greater distance between the intubator and the patient’s airway. Videolaryngoscopy may also increase chances of first attempt success.31,32 If first pass attempt at intubation is not successful, two-person BMV technique should be used to optimize the chance for obtaining a tight seal during re-oxygenation and to reduce the risk of exhaled air leakage during manual ventilation in preparation for repeat intubation attempt.29,33 Table 3 provides some recommendations for clinicians performing manual ventilation and intubation in patients with COVID-19.
Mechanical Ventilation
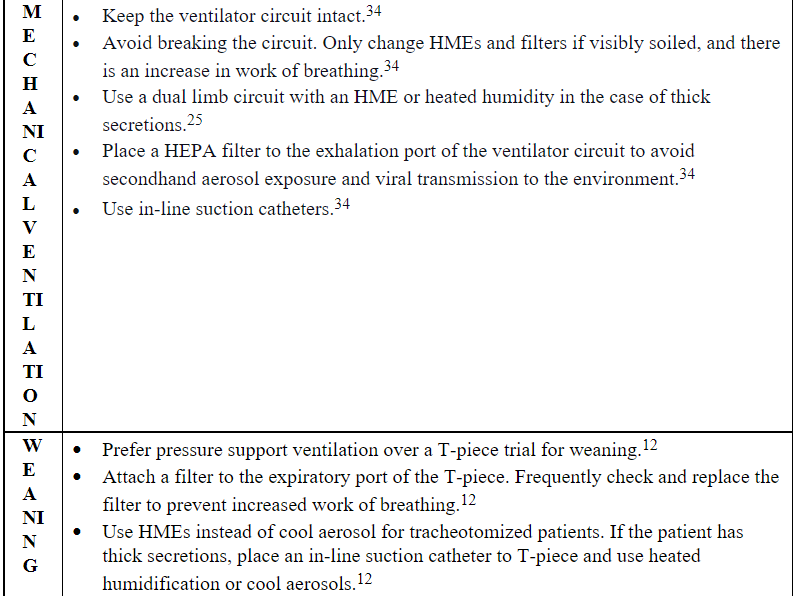
Patients with severe COVID-19 are at risk for severe pneumonia or sepsis that often requires mechanical ventilation.34 Providing effective humidification and lung protective ventilation while protecting HCPs from potential exposure to aerosols is crucial in the era of COVID-19. Lin et al25 recommend that patients requiring mechanical ventilation are placed on a dual limb circuit with an HME to limit the amount of contaminated condensate that can be produced if the patient is disconnected from the ventilator. One concern with using HMEs is the allowance of up to 60% of medical aerosols to pass through the filter.34 Therefore, it is recommended that a HEPA or a pleated hydrophobic filter be placed after the expiratory limb of the ventilator circuit to reduce the exhaled pathogens from intubated patients receiving mechanical ventilation.34
Patients with thick tenacious secretions or patients that are unable to tolerate the additional dead space added to the circuit from the HME should be placed on a heated humidified circuit with a pleated hydrophobic filter placed at the expiratory side of the ventilator. One disadvantage to using heated humidified circuits is the large volume of condensate that is generated by the circuit due to the sensitivity of the circuit to changes in environmental temperature. HCPs should follow aerosol precautions and don appropriate PPE when working with mechanically ventilated patients to minimize the risk of exposure to aerosols when changing filters or disconnecting the ventilator circuit. Ventilator circuits and filters should only be changed when visibly soiled and the end of the circuit should be capped immediately after the following disconnection. If a disconnection is needed, the endotracheal tube should be clamped, and the ventilator placed in standby mode. To reconnect the circuit, clinicians should attach and tighten all circuit connections and unclamp endotracheal tube before resuming mechanical ventilation.34 Clamping the endotracheal tube in passively ventilated patients is recommended to avoid alveolar derecruitment and to reduce the potential spread of bioaerosols.12
Weaning and Extubation
Although T-piece trial is commonly used for weaning, it opens the patient’s airway to the environment and increases the risk of exposure and infection to HCPs. Therefore, pressure support ventilation is a preferred method of weaning in ventilator-dependent patients during the COVID-19 pandemic. If the T-piece needs to be used, clinicians should attach a filter to the exhalation port of the T-piece to protect HCPs and use the T-piece with a closed-suction catheter and humidified oxygen delivery device to keep the circuit intact.12 It is also vital to check the filter frequently and replace it when necessary to prevent filter clogging that may cause an increase in work of breathing.
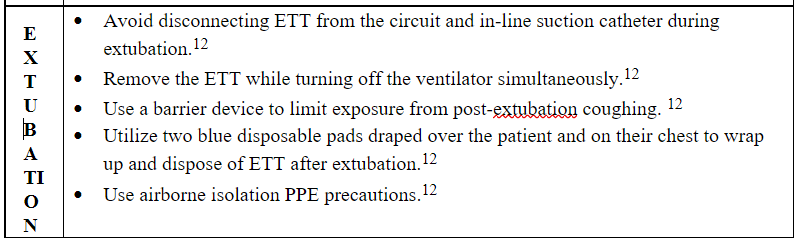
Extubation induces cough that may increase the dispersion of contaminated bioaerosols to the environment and the risk of viral transmission among patients and HCPs. Clinicians should not disconnect the endotracheal tube from the ventilator and the closed suction catheter to decrease the risk of exposure and infection.12 It is also essential to follow airborne isolation PPE precautions and consider using a barrier device to limit exposure from post-extubation coughing. Using two blue disposable pads draped over the patient and on their chest to wrap up and dispose of endotracheal tube after extubation is recommended (Table 3).
Bronchoscopy Assist
Since bronchoscopy is a high-risk exposure and infection for HCPs and patients,35 it is vital to defer bronchoscopy in non-urgent cases. In urgent emergency indications, clinicians should assess the need for each procedure case-by-case and use PPE to minimize the risk of exposure and infection for HCPs. Also, performing bronchoscopy during this pandemic needs to take into account some recommendations such as those proposed in Table 2. For instance, it is essential to implement full barrier precautions and restrict the number of personnel performing the bronchoscopy. Clinicians should perform the bronchoscopy in negative pressure rooms, use adequate sedation to avoid coughing, place a surgical mask on the patient’s face and make a slot on the mask to introduce the bronchoscope.36,37 Whereas a single-use flexible bronchoscope is recommended to avoid cross-contamination and accidental transmission, high-level disinfection procedures should be utilized for re-usable bronchoscopes.36,37 The insertion location of the bronchoscope is patient specific. While nasal access should be preferred in spontaneously breathing patients, a swivel adapter or an NIV mask with an examination port could be used to insert the bronchoscope for patients receiving mechanical ventilation or NIV, respectively.12 Clinicians should also limit the use of atomizers, nebulizers, jet ventilation during bronchoscopy.36,37,38
Gaps in the Literature
Unanticipated PPE shortages and lack of understanding about the viral transmissions during this global pandemic have led to new devices, tools, and interventions related to AGPs. Data specific to AGPs in the era of COVID-19 are warranted. For instance, there is a significant research gap regarding the effectiveness of these new devices and interventions to reduce viral transmission in healthcare settings. Their cost and resource implications for decreasing the transmission of COVID-19 and the social and cultural factors that may affect the compliance of HCPs with the use of these new devices and interventions are needed. Also, it is essential to understand the epidemiology of viral transmission with these new technologies and procedures in health care settings. While laboratory studies elucidate the effectiveness of AGPs in decreasing exhaled air dispersion into the environment, it is difficult to differentiate medical aerosols from bioaerosols in these studies. Therefore, the findings of laboratory studies should be validated by randomized clinical trials. For instance, although filters are commonly used with nebulizers, it is not known how often they need to be replaced in patients with COVID-19. Therefore, clinicians should check the filters frequently and change them regularly to avoid increased airway resistance and work of breathing. National and international professional societies should collaborate with expert scientists to develop pertinent research endeavors that will enable the development of evidence-based guidelines on AGPs. The suggestions in this document are based on the scientific evidence available at the time of publication.
Conclusion
HCPs performing AGPs are at risk of aerosol-transmitted infections. Given the issue with viral transmission and the importance of infection control and prevention, the COVID-19 pandemic demands serious precautions for AGPs. Therefore, the utility of each AGP must be evaluated carefully, and HCPs should make a multi-disciplinary decision on “when, how, and why” to perform an AGP to reduce the risk of exposure and viral transmission in the era of COVID-19. Clinical studies specific to AGPs during this pandemic are urgently needed. This paper emphasizes the need for AGP-specific protocols, evidence-based guidelines, and training programs to lessen exposure and create a safer environment for HCPs. Through the pragmatic recommendations on AGPs explained in this article and the infection control and prevention guidelines published by the CDC and WHO, clinicians can control the spread of infection in their institutions and ensure the well-being of themselves, their patients, and caregivers. For a pragmatic recommendation summary, refer to Tables 1-3.
Tables
Table 1. Pragmatic recommendations for clinicians using aerosol therapy, and oxygen therapy, in the era of COVID-19.
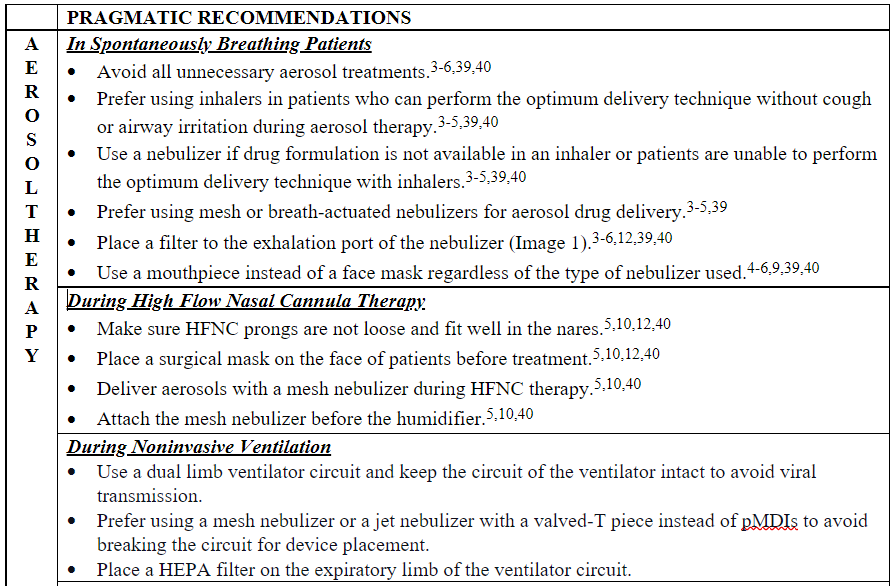

Table 2. Pragmatic recommendations for clinicians using bronchoscopy, high flow nasal cannula (HFNC) therapy, and non–invasive ventilation (NIV) in the era of COVID-19.
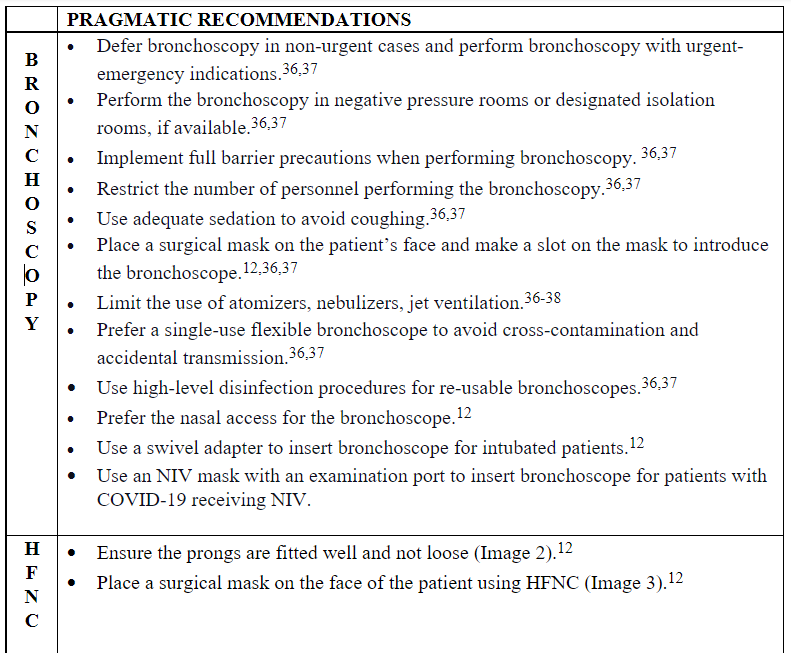
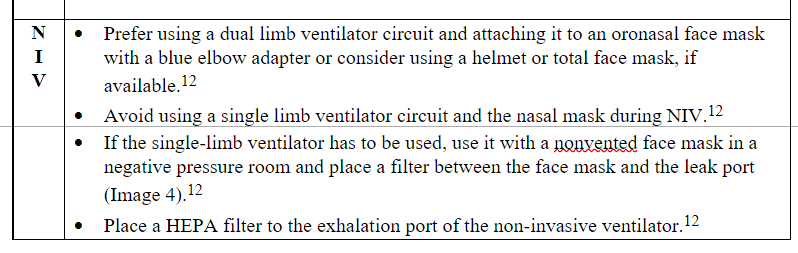
Table 3. Pragmatic recommendations on intubation, mechanical ventilation, weaning, and extubation in the era of COVID-19.

References
- Klompas M, Baker M, Rhee C. What Is an Aerosol-Generating Procedure? JAMA Surg 2021;156(2):113-114.
- Morawska L, Johnson GR, Ristovski ZD, Hargreaves M, Mengersen K, Corbett S, et al. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. Journal of Aerosol Science 2009;40(3):256-269.
- Ari A. Use of aerosolised medications at home for COVID-19. Lancet Respir Med 2020;8(8):754-756.
- Ari A. Promoting Safe and Effective Use of Aerosol Devices in Covid-19:Risks and Suggestions for Viral Transmission. Expert Opin Drug Deliv 2020;17(11):1509-1513.
- Ari A. Practical strategies for a safe and effective delivery of aerosolized medications to patients with COVID-19. Respir Med 2020;167:105987.
- Fink JB, Ehrmann S, Li J, Dailey P, McKiernan P, Darquenne C, et al. Reducing Aerosol-Related Risk of Transmission in the Era of COVID-19: An Interim Guidance Endorsed by the International Society of Aerosols in Medicine. J Aerosol Med Pulm Drug Deliv 2020;33(6):300-304.
- Hui DS, Chow BK, Chu LCY, Ng SS, Hall SD, Gin T, et al. Exhaled air and aerosolized droplet dispersion during application of a jet nebulizer. Chest 2009;135(3):648-654.
- Tang JW, Kalliomaki P, Varila TM, Waris M, Koskela H. Nebulisers as a potential source of airborne virus. J Infect 2020;81(4):647-679.
- McGrath JA, O’Sullivan A, Bennett G, O’Toole C, Joyce M, Byrne MA, et al. Investigation of the Quantity of Exhaled Aerosols Released into the Environment during Nebulisation. Pharmaceutics 2019;11(2).
- Ari A, Moody GB. How to deliver aerosolized medications through high flow nasal cannula safely and effectively in the era of COVID-19 and beyond: A narrative review. Canadian Journal of Respiratory Therapy 2021;57:22-25.
- Ari A, Fink J, Pilbeam S. Secondhand aerosol exposure during mechanical ventilation with and without expiratory filters: An in-vitro study. Indian Journal of Respiratory Care 2016;5(1):677-682.
- Kaur R, Weiss TT, Perez A, Fink JB, Chen R, Luo F, et al. Practical strategies to reduce nosocomial transmission to healthcare professionals providing respiratory care to patients with COVID-19. Crit Care 2020;24(1):571.
- Bem RA, van Mourik N, Klein-Blommert R, Spijkerman IJ, Kooij S, Bonn D, et al. Risk of Aerosol Formation During High-Flow Nasal Cannula Treatment in Critically Ill Subjects. Respir Care 2021;66(6):891-896.
- Rali A, Garies T, Narendra D, Patel P, Guntupalli K. High-flow nasal cannula: COVID 19 and beyond. Indian Journal of Respiratory Care 2020;9(2):134-140.
- Chen WQ, Ling WH, Lu CY, Hao YT, Lin ZN, Ling L, et al. Which preventive measures might protect health care workers from SARS? BMC Public Health 2009;9:81.
- Fowler RA, Guest CB, Lapinsky SE, Sibbald WJ, Louie M, Tang P, et al. Transmission of Severe Acute Respiratory Syndrome during Intubation and Mechanical Ventilation. American Journal of Respiratory and Critical Care Medicine 2004;169(11):1198-1202.
- Loeb M, McGeer A, Henry B, Ofner M, Rose D, Hlywka T, et al. SARS among critical care nurses, Toronto. Emerging infectious diseases 2004;10(2):251-255.
- Ma H-j, Wang H-w, Fang L-q, Jiang J-f, Wei M-t, Liu W, et al. [A case-control study on the risk factors of severe acute respiratory syndromes among health care workers]. Zhonghua liu xing bing xue za zhi = Zhonghua liuxingbingxue zazhi 2004;25(9):741-744.
- Pei L-y, Gao Z-c, Yang Z, Wei D-g, Wang S-x, Ji J-m, et al. Investigation of the influencing factors on severe acute respiratory syndrome among health care workers. Beijing da xue xue bao Yi xue ban = Journal of Peking University Health sciences 2006;38(3):271-275.
- Raboud J, Shigayeva A, McGeer A, Bontovics E, Chapman M, Gravel D, et al. Risk factors for SARS transmission from patients requiring intubation: a multicentre investigation in Toronto, Canada. PLoS One 2010;5(5):e10717.
- Scales DC, Green K, Chan AK, Poutanen SM, Foster D, Nowak K, et al. Illness in intensive care staff after brief exposure to severe acute respiratory syndrome. Emerging infectious diseases 2003;9(10):1205-1210.
- Teleman MD, Boudville IC, Heng BH, Zhu D, Leo YS. Factors associated with transmission of severe acute respiratory syndrome among healthcare workers in Singapore. Epidemiology and Infection 2004;132(5):797-803.
- Wong T-w, Lee C-k, Tam W, Lau JT-f, Yu T-s, Lui S-f, et al. Cluster of SARS among medical students exposed to single patient, Hong Kong. Emerging infectious diseases 2004;10(2):269-276.
- Tran K, Cimon K, Severn M, Pessoa-Silva CL, Conly J. Aerosol generating procedures and risk of transmission of acute respiratory infections to healthcare workers: a systematic review. PLoS One 2012;7(4):e35797.
- Organization WH. Infection prevention and control during health care when coronavirus disease (COVID-19) is suspected or confirmed: Interim guidance
- Weissman DN, de Perio MA, Radonovich LJ, Jr. COVID-19 and Risks Posed to Personnel During Endotracheal Intubation. JAMA 2020;323(20):2027-2028.
- Chan MTV, Chow BK, Lo T, Ko FW, Ng SS, Gin T, et al. Exhaled air dispersion during bag-mask ventilation and sputum suctioning – Implications for infection control. Sci Rep 2018;8(1):198.
- Edelson DP, Sasson C, Chan PS, Atkins DL, Aziz K, Becker LB, et al. Interim Guidance for Basic and Advanced Life Support in Adults, Children, and Neonates With Suspected or Confirmed COVID-19: From the Emergency Cardiovascular Care Committee and Get With The Guidelines-Resuscitation Adult and Pediatric Task Forces of the American Heart Association. Circulation 2020;141(25):e933-e943.
- Cook TM, El-Boghdadly K, McGuire B, McNarry AF, Patel A, Higgs A. Consensus guidelines for managing the airway in patients with COVID-19: Guidelines from the Difficult Airway Society, the Association of Anaesthetists the Intensive Care Society, the Faculty of Intensive Care Medicine and the Royal College of Anaesthetists. Anaesthesia 2020;75(6):785-799.
- Yao W, Wang T, Jiang B, Gao F, Wang L, Zheng H, et al. Emergency tracheal intubation in 202 patients with COVID-19 in Wuhan, China: lessons learnt and international expert recommendations. Br J Anaesth 2020;125(1):e28-e37.
- Hall D, Steel A, Heij R, Eley A, Young P. Videolaryngoscopy increases ‘mouth-to-mouth’ distance compared with direct laryngoscopy. Anaesthesia 2020;75(6):822-823.
- Schumacher J, Arlidge J, Dudley D, Sicinski M, Ahmad I. The impact of respiratory protective equipment on difficult airway management: a randomised, crossover, simulation study. Anaesthesia 2020;75(10):1301-1306.
- Montrief T, Ramzy M, Long B, Gottlieb M, Hercz D. COVID-19 respiratory support in the emergency department setting. Am J Emerg Med 2020;38(10):2160-2168.
- Lin HL, Fink JB, Tsai YH, Wan GH. Managing humidity support in intubated ventilated patients with coronavirus disease 2019 (COVID-19). Infection Control & Hospital Epidemiology 2020:1-2.
- Thompson KA, Pappachan JV, Bennett AM, Mittal H, Macken S, Dove BK, et al. Influenza aerosols in UK hospitals during the H1N1 (2009) pandemic–the risk of aerosol generation during medical procedures. PLoS ONE [Electronic Resource] 2009;8(2):e56278.
- Lentz RJ, Colt H. Summarizing societal guidelines regarding bronchoscopy during the COVID-19 pandemic. Respirology 2020;25(6):574-577.
- Wahidi MM, Shojaee S, Lamb CR, Ost D, Maldonado F, Eapen G, et al. The Use of Bronchoscopy During the Coronavirus Disease 2019 Pandemic: CHEST/AABIP Guideline and Expert Panel Report. Chest 2020;158(3):1268-1281.
- Nitesh J, Kashyap R, Surani SR. What we learned in the past year in managing our COVID-19 patients in intensive care units? World J Crit Care Med 2021;10(4):81-101.
- Ari A, Blain K, Soubra S, Hanania N. Treating COPD Patients with Aerosolized Medications in The Era Of COVID-19: Options and Rationales for Patients at Home. International Journal of Chronic Obstructive Pulmonary Diseases 2021; 16: 2687-2695.
- Ari A, Scott JB. Lessons learned about aerosol drug delivery in the era of COVID-19. CHEST 2021. Available at https://chestnet.org/Topic-Collections/COVID-19/COVID-in-Focus/Lessons-Learned-About-Aerosol-Drug-Delivery-in-the-Era-of-COVID-19
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.