In the United States, we care for more than four million patients in an ICU each year; and at any given time, approximately 40% are receiving invasive mechanical ventilation.1,2 When considering monitoring mechanically ventilated patients, we must contemplate three questions:
- Why are we monitoring?
- How good are the tools we use to monitor?
- Does monitoring lead to a change in management that impacts outcomes?
With any patient monitoring system or parameter, our general goal is to identify early warning signs that allow us to reduce or prevent patient harm. This goal is crucial during mechanical ventilation, as almost everything we do can cause patient harm.
Respiratory therapists are the front-line care providers for patients requiring mechanical ventilation, especially those in intensive care units or other chronic ventilator units. We will focus on the rationale, accuracy, and effectiveness of preventing harm for each of these monitoring modalities. Monitoring includes both non-ventilator-specific parameters as well as ventilator monitoring.
Non-ventilator Monitoring
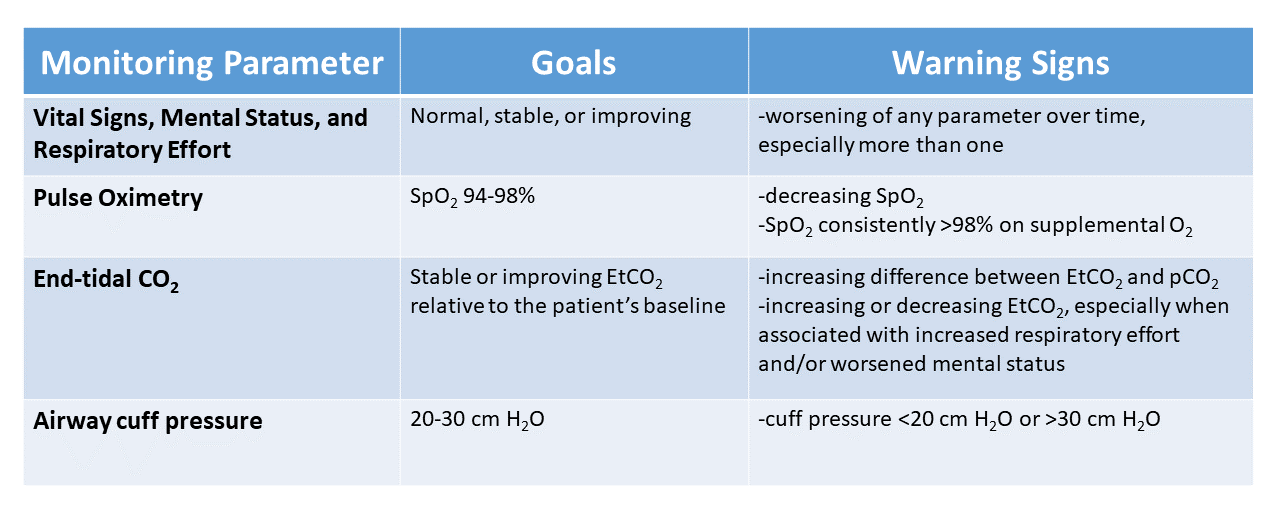
Vital signs, mental status, and respiratory effort
With all the advanced monitoring equipment we use, we must not overlook the importance of a focused clinical exam performed by a skilled respiratory therapist. Depending on the patient care setting and patient acuity, we may perform this almost continuously or as infrequently as once per shift.
We must take care not to perform each assessment in isolation but rather to evaluate whether the patient’s status has changed over the last several minutes, hours, or even days. For example, a therapist detecting a change in mental status with a slight increase in respiratory rate may be a critical early clue to a patient deteriorating. Both of these abnormalities are criteria for early detection of sepsis.
In addition to vital signs and mental status, we must closely monitor the patient’s respiratory effort or work of breathing. An increase in respiratory effort could be a clue to patient-ventilator dyssynchrony, acidemia, pneumothorax, or other pulmonary or non-pulmonary abnormality. While not immediately apparent as to what is causing the problem, recognizing when something is not quite right is essential and allows for earlier evaluation and treatment.
Pulse oximetry
Pulse oximetry allows us to continuously and noninvasively monitor the arterial hemoglobin saturation (SpO2) in patients who may have rapidly changing clinical conditions due to respiratory failure. It identifies early warning signs of changes in respiratory status and ensures hypoxemic patients receive appropriate supplemental oxygen.
We want to ensure we provide adequate supplemental oxygen to prevent hypoxemia and reduce the risk for long-term neuropsychological impairment.3 On the other end, if we provide patients with excess supplemental oxygen and maintain high SpO2 levels, they may have significant hyperoxia that goes unrecognized without arterial sampling of the PaO2. This is important to recognize because providing supplemental oxygen to maintain an SpO2 of 98-100% also can worsen outcomes, even when only maintained for short periods of time.4,5 Therefore, targeting an SpO2 of 94-98% in most patients requiring mechanical ventilation best balances the risks of both hypoxia and hyperoxia.6
End-tidal CO2
If we do not provide adequate ventilation to patients, they can develop hypercarbia, leading to respiratory acidosis and cardiovascular collapse. Significant hypercarbia may not cause desaturation on pulse oximetry until very late in a patient receiving supplemental oxygen or manifest as a change in mental status if the patient receives sedative medications. End-tidal CO2 (EtCO2), which provides a measurement of CO2 concentration in exhaled gas, can help us monitor the adequacy of perfusion and ventilation. We commonly use EtCO2 to confirm proper endotracheal tube placement, assess the quality and effectiveness of cardiopulmonary resuscitation, and monitor for ventilation changes.
We can generally expect EtCO2 to be an acceptable estimate of alveolar PCO2 (PACO2) in normal subjects; however, the difference between the EtCO2 and PACO2 can be quite large in patients with diseased lungs. Since many of the patients we care for on mechanical ventilators have diseases that affect either the pulmonary parenchyma or the pulmonary vasculature, they often have significantly increased alveolar dead space, which leads to a greater difference between PACO2 and EtCO2. In this case, the trend of the EtCO2 may be more useful that the absolute value and a blood gas may be required periodically to assess the difference between EtCO2 and PCO2 in the blood.
EtCO2 can be useful in weaning mechanical ventilation and initiation of trials off the ventilator in patients with tracheostomies. EtCO2 can serve as one additional safety monitor to warn when a patient is failing liberation or weaning from mechanical ventilation. Finally, volumetric capnography, which plots the partial pressure of exhaled CO2 against exhaled tidal volume allows for more accurate calculation of dead space and CO2 production, but it is not readily available in most settings.7
Airway cuff pressure
We monitor the airway cuff pressure on an endotracheal or tracheostomy tube for two main reasons: maintain airway pressure during mechanical ventilation and prevent leakage of secretions into the lungs. If we overinflate the cuff, then we may be applying focal pressure on the tracheal mucosa that impedes capillary blood flow, leading to ischemia of the tissue. This injury can cause long-term complications, such as vocal cord paralysis and tracheal stenosis. Unfortunately, even modern airway cuffs that use a larger volume to distribute the pressure more evenly across the airway mucosa and achieve a seal with a much lower pressure do not eliminate this risk.8
Maintaining the upper range of airway cuff pressures less than 30 cm H2O does not exceed the capillary perfusion pressure and can help reduce pressure-related ischemia and injury to the trachea. Therefore, we cannot wholly prevent secretion drainage solely by increasing airway cuff pressures. We do, however, see an apparent increase in leakage of fluid around the cuff and ventilator-associated pneumonia at a cuff pressure of <15-20 cm H2O.9,10 For these reasons, it is essential to monitor airway cuff pressures and maintain an airway cuff pressure of 20-30 cm of H2O.
We often assess cuff pressures using varying methods and at varying frequencies depending on our clinical settings and institutional protocols. While the pilot balloon palpation technique and the minimal occlusive pressure test are common and simple to perform at the bedside, even skilled providers typically inflate cuffs to pressures well above what is generally considered safe. Additionally, cuff pressures tend to decrease over time. Therefore, continuous or frequent monitoring and adjustment of airway cuff pressure appears to be the best method to maintain airway cuff pressure within the target range to reduce airway complications and ventilator-associated pneumonia.
Ventilator Monitoring
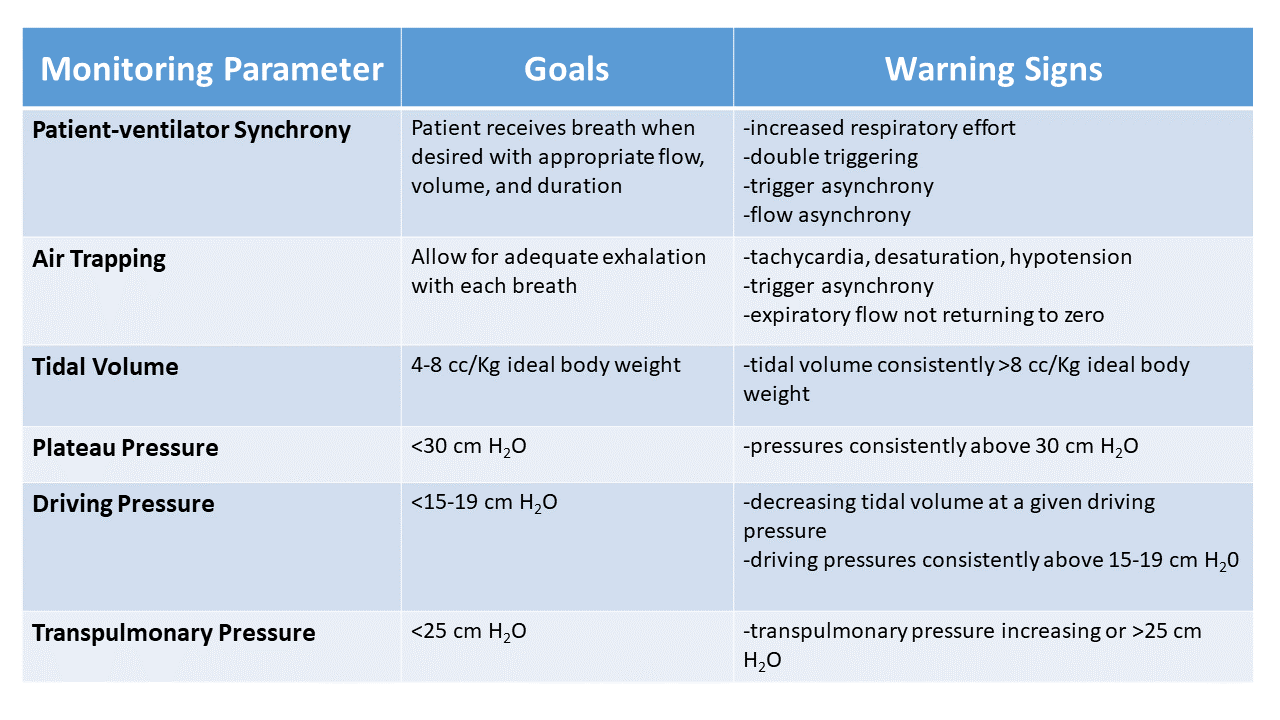
Patient-ventilator synchrony
Along with our clinical assessment mentioned earlier, we want to see that the patient is interacting seamlessly with the ventilator. Patient-ventilator synchrony can be most simply defined as the patient receiving the volume of air and flow that they desire exactly when they want it for only as long as they want it. Poor patient-ventilator synchrony can lead to increased work of breathing, prolonged duration of mechanical ventilation, and increased diaphragmatic fatigue and weakness.
Patient-ventilator dyssynchrony can be challenging to detect and requires that we simultaneously assess the patient and the ventilator waveforms. First, we want to see that when the patient generates an effort, they quickly receive the breath. Second, we want to ensure that the flow is adequate to meet their demand, as demonstrated by smooth pressure and flow vs. time waveforms. And finally, we want to see that they are not working to exhale against the ventilator or receiving an immediate second breath because the inspiratory time or volume were not appropriate. Treating patient-ventilator dyssynchrony can be as simple as slightly increasing the inspiratory time, or it may require multiple ventilator changes and adjustments to patient sedation.
Air trapping
The concept of air trapping is especially important when managing patients with acute exacerbations of obstructive lung disease who have a prolonged expiratory phase. When the respiratory rate is too rapid to adequately allow exhalation before the next breath is delivered, air is trapped within the lungs. This trapping leads to hyperinflation, difficulty in triggering spontaneous breaths, worsened V/Q mismatch, and even cardiovascular collapse from increased intrathoracic pressure.
We can most easily identify air trapping by looking at the expiratory portion of the flow vs. time graphic on the ventilator. If the flow is not returning to zero or at least plateauing, some degree of air trapping is likely present. Air trapping can be assessed by adjusting the respiratory rate and monitoring the effects on airway pressure or tidal volume. In a patient on volume control ventilation air trapping manifests as increasing peak and plateau pressure as the respiratory rate is increased. In a pressure control mode of ventilation, the tidal volumes will progressively decrease as the respiratory rate is increased. When this is seen, the respiratory rate should typically decrease until we no longer see a tidal volume or airway pressure change.
Tidal volume
Of all the parameters we monitor, ensuring that patients receive low tidal volumes (4-8 cc/Kg of ideal body weight) is the one intervention that has the most substantial evidence supporting it across all settings where patients receive invasive mechanical ventilation.11 Tidal volumes are a simple parameter to monitor, and we should take care to define what constitutes a low tidal volume in each of our patients.
When patients consistently receive tidal volumes outside of the acceptable range, we must work together as respiratory therapists and physicians to delineate an acceptable plan for the patient. This will involve identifying and treating the underlying cause (i.e., pain or acidosis), adjusting the ventilator as needed, and weighing the risks of increased sedation/neuromuscular blockade vs. higher tidal volumes depending on the underlying disease process. While identifying high tidal volumes is easy, effectively achieving lower tidal volumes while also mitigating the risks of these other interventions can be challenging and requires continual monitoring, adjustment, and teamwork.
Plateau pressure
Plateau pressure typically refers to the end-inspiratory airway plateau pressure measured using an inspiratory hold maneuver in a patient who is not generating an effort to breathe. This airway pressure estimates the lungs’ distending pressure when receiving the given tidal volume. It is generally accepted that the plateau pressure be maintained at <30 cm H20 in most mechanically ventilated patients.12
Measuring the plateau pressure requires that the inspiratory hold maneuver be sufficiently long for the airflow within the lungs to stop (i.e., airway pressure equals alveolar pressure) before the patient generates any inspiratory or expiratory effort. An increasing plateau pressure at a given tidal volume and PEEP indicates that the lung compliance is decreasing, which may indicate worsening lung disease. Therefore, we monitor plateau pressure to ensure we are not exceeding safe pressure targets and as an early warning sign of progression of lung injury.
Driving pressure
Driving pressure can be considered the pressure required to drive a given tidal volume into a patient’s lungs and is inversely related to lung compliance. In other words, if lung compliance decreases, then driving pressure will have to increase to achieve the same tidal volume. We measure driving pressure by subtracting the end-inspiratory plateau pressure from the PEEP.
Driving Pressure = Plateau pressure – PEEP
In adult patients with severe ARDS, the aerated portion of the lung can be very small, even giving rise to the term baby lung.13 In this case, it would make sense that targeting a tidal volume that matches the functioning aerated lung rather than an ideal normal lung may be the best approach to reduce excessive regional strain on the lung and ventilator-induced lung injury. A simple strategy to achieve this is by using lung compliance (or driving pressure) to scale the tidal volume. High driving pressures may be as significant as large tidal volumes in causing ventilator-induced lung injury. Even a normal tidal volume of 6-8 ml/kg ideal body weight may be excessive if the driving pressure is excessive (e.g.,>15-19 cm H2O).14,15,16 We should routinely monitor to ensure patients are not being ventilated with excessively high driving pressures, especially in those with severely injured lungs.
Transpulmonary pressure
The end-inspiratory plateau pressure and PEEP are measurements of airway pressure, but we use them as surrogates for the alveolar pressure. However, the pressure we care about is the alveolar distending pressure or transpulmonary pressure. This pressure determines alveolar hyperinflation and alveolar collapse. We calculate the transpulmonary pressure as the difference between airway pressure and pleural pressure and allows us to delineate the pressure delivered to the lung from the one acting to simply move the chest wall and abdomen.
Pleural pressure cannot be measured by the ventilator alone. We use a pressure-transducing catheter inserted either orally or nasally into the esophagus to measure pleural pressure, allowing us to calculate transpulmonary pressure.
Transpulmonary Pressure = Airway Pressure – Pleural Pressure
Or – Transpulmonary Pressure = Airway Pressure – Esophageal Pressure 17
Airway plateau pressures are adequate for most patients. Still, differences between airway plateau and transpulmonary plateau pressures are most pronounced in obese patients and those with restrictive chest wall or pleural space disorders.18
The other group of patients where monitoring transpulmonary pressures can be critical are those with excess spontaneous effort during positive-pressure ventilation. This can lead to significantly negative pleural pressures, high transpulmonary pressures, and increased risk of Self-Induced Lung Injury (SILI).19,20 All the while, the airway pressures may appear well within an acceptable range.
While these potential benefits to monitoring transpulmonary pressure with the help of an esophageal catheter are theoretically attractive, available evidence does not support the routine use of esophageal pressure measurements to guide clinical care. On the other hand, though, it may be useful in select patients with severe obesity, significant abnormalities in chest wall compliance, or significant patient effort of breathing.
Summary
Mechanical ventilation is a prevalent form of life support used worldwide to support patients with respiratory failure. Unfortunately, while potentially life-saving, it carries with it the potential for significant harm even when provided for only short periods. Therefore, it is imperative that patients receiving mechanical ventilation be monitored closely to reduce the risk of injury. This practice involves a skilled respiratory therapist monitoring both non-ventilator and ventilator-specific parameters.
References
- Wunsch H, Wagner J, Herlim M, Chong DH, Kramer AA, Halpern SD. ICU occupancy and mechanical ventilator use in the United States. Crit Care Med 2013;41(12):2712-2719
- Mikkelsen ME, Christie JD, Lanken PN, Biester RC, Thompson BT, Bellamy SL, et al. The adult respiratory distress syndrome cognitive outcomes study: long-term neuropsychological function in survivors of acute lung injury. Am J Respir Crit Care Med 2012;185(12):1307-1315.
- Girardis M, Busani S, Damiani E, Donati A, Rinaldi L, Marudi A, et al. Effect of Conservative vs Conventional Oxygen Therapy on Mortality Among Patients in an Intensive Care Unit: The Oxygen-ICU Randomized Clinical Trial. JAMA 2016;316(15):1583-1589.
- Kilgannon JH, Jones AE, Shapiro NI, Angelos MG, Milcarek B, Hunter K, et al. Association between arterial hyperoxia following resuscitation from cardiac arrest and in-hospital mortality. JAMA 2010;303(21):2165-2171.
- Rackley CR. Monitoring During Mechanical Ventilation. Respir Care 2020;65(6):832-846.
- Verscheure S, Massion PB, Verschuren F, Damas P, Magder S. Volumetric capnography: lessons from the past and current clinical applications. Crit Care 2016;20(1):184.
- Mathias DB, Wedley JR. The effects of cuffed endotracheal tubes on the tracheal wall. Br J Anaesth 1974;46(11):849-852.
- Nseir S, Zerimech F, Fournier C, Lubret R, Ramon P, Durocher A, et al. Continuous control of tracheal cuff pressure and microaspiration of gastric contents in critically ill patients. Am J Respir Crit Care Med 2011;184(9):1041-1047.
- Young PJ, Rollinson M, Downward G, Henderson S. Leakage of fluid past the tracheal tube cuff in a benchtop model. Br J Anaesth 1997;78(5):557-562.
- Rackley CR, MacIntyre NR. Low Tidal Volumes for Everyone? Chest 2019.
- Fan E, Del Sorbo L, Goligher EC, Hodgson CL, Munshi L, Walkey AJ, et al. An Official American Thoracic Society/European Society of Intensive Care Medicine/Society of Critical Care Medicine Clinical Practice Guideline: Mechanical Ventilation in Adult Patients with Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med 2017;195(9):1253-1263.
- Gattinoni L, Pesenti A. The concept of “baby lung”. Intensive Care Med 2005;31(6):776-784.
- Aoyama H, Pettenuzzo T, Aoyama K, Pinto R, Englesakis M, Fan E. Association of Driving Pressure With Mortality Among Ventilated Patients With Acute Respiratory Distress Syndrome: A Systematic Review and Meta-Analysis. Crit Care Med 2018;46(2):300-306.
- Villar J, Martin-Rodriguez C, Dominguez-Berrot AM, Fernandez L, Ferrando C, Soler JA, et al. A Quantile Analysis of Plateau and Driving Pressures: Effects on Mortality in Patients With Acute Respiratory Distress Syndrome Receiving Lung-Protective Ventilation. Crit Care Med 2017;45(5):843-850.
- Amato MB, Meade MO, Slutsky AS, Brochard L, Costa EL, Schoenfeld DA, et al. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med 2015;372(8):747-755.
- Akoumianaki E, Maggiore SM, Valenza F, Bellani G, Jubran A, Loring SH, et al. The application of esophageal pressure measurement in patients with respiratory failure. Am J Respir Crit Care Med 2014;189(5):520-531.
- Owens RL, Campana LM, Hess L, Eckert DJ, Loring SH, Malhotra A. Sitting and supine esophageal pressures in overweight and obese subjects. Obesity (Silver Spring) 2012;20(12):2354-2360.
- Brochard L, Slutsky A, Pesenti A. Mechanical Ventilation to Minimize Progression of Lung Injury in Acute Respiratory Failure. Am J Respir Crit Care Med 2017;195(4):438-442.
- Yoshida T, Fujino Y, Amato MB, Kavanagh BP. Fifty Years of Research in ARDS. Spontaneous Breathing during Mechanical Ventilation. Risks, Mechanisms, and Management. Am J Respir Crit Care Med 2017;195(8):985-992.
- Barrett ML SM, Elixhauser A, Honigman LS, Pines JM. Utilization of intensive care services, 2011. Statistical Brief #185. Healthcare Cost and Utilization Project. Agency for Healthcare Research and Quality. 2014.
Email newsroom@aarc.org with questions or comments, we’d love to hear from you.